Neumora Therapeutics IPO Presentation Deck
NMRA-511 Demonstrates Relevant Pharmacodynamics with the Potential to Address
Anxiety Disorders
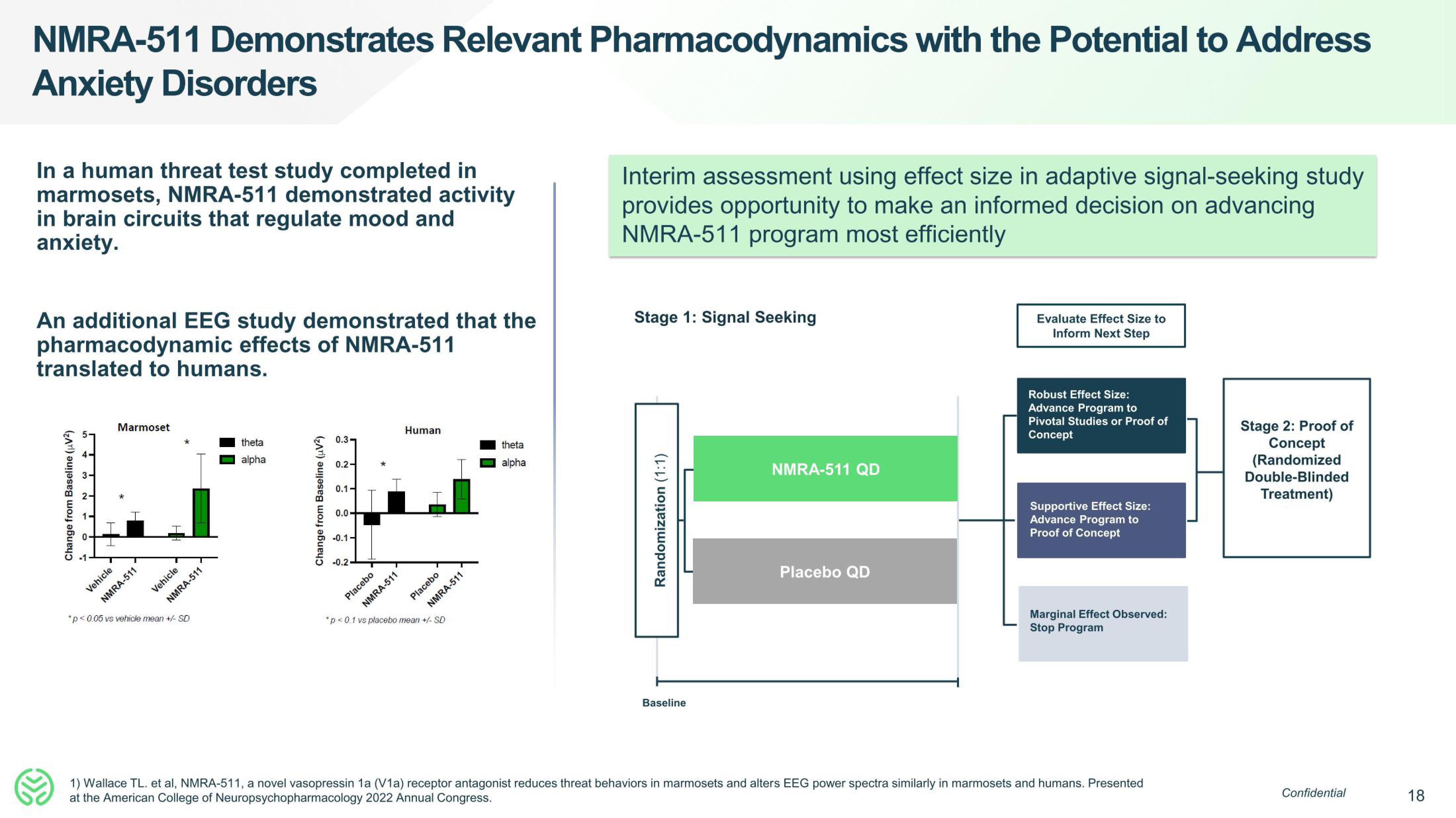
In a human threat test study completed in
marmosets, NMRA-511 demonstrated activity
in brain circuits that regulate mood and
anxiety.
An additional EEG study demonstrated that the
pharmacodynamic effects of NMRA-511
translated to humans.
Change from Baseline (V²)
Marmoset
Vehicle
NMRA-511
Vehicle
*p<0.05 vs vehicle mean +/- SD
NMRA-511
theta
alpha
Change from Baseline (uV²)
0.3-
0.2-
0.1-
0.0-
-0.1-
-0.2
Placebo
NMRA-511
Human
T
Placebo
NMRA-511
*p<0.1 vs placebo mean +/- SD
theta
alpha
Interim assessment using effect size in adaptive signal-seeking study
provides opportunity to make an informed decision on advancing
NMRA-511 program most efficiently
Stage 1: Signal Seeking
Randomization (1:1)
Baseline
NMRA-511 QD
Placebo QD
Evaluate Effect Size to
Inform Next Step
Robust Effect Size:
Advance Program to
Pivotal Studies or Proof of
Concept
Supportive Effect Size:
Advance Program to
Proof of Concept
Marginal Effect Observed:
Stop Program
1) Wallace TL. et al, NMRA-511, a novel vasopressin 1a (V1a) receptor antagonist reduces threat behaviors in marmosets and alters EEG power spectra similarly in marmosets and humans. Presented
at the American College of Neuropsychopharmacology 2022 Annual Congress.
Stage 2: Proof of
Concept
(Randomized
Double-Blinded
Treatment)
Confidential
18View entire presentation