Aeglea BioTherapeutics Investor Presentation Deck
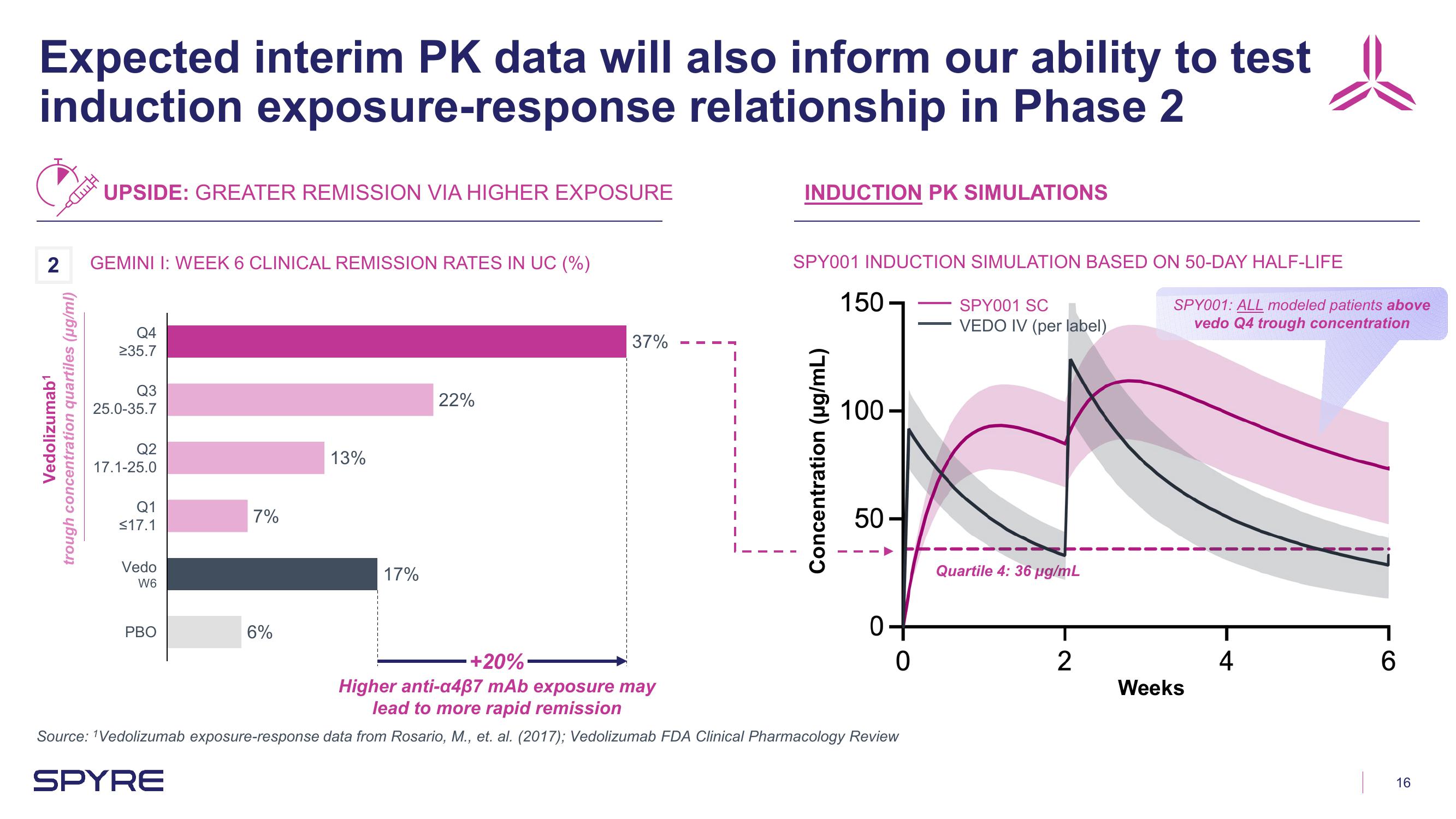
Expected interim PK data will also inform our ability to test
induction exposure-response relationship in Phase 2
UPSIDE: GREATER REMISSION VIA HIGHER EXPOSURE
2 GEMINI I: WEEK 6 CLINICAL REMISSION RATES IN UC (%)
Vedolizumab¹
trough concentration quartiles (µg/ml)
38%
Q4
≥35.7
Q3
25.0-35.7
Q2
17.1-25.0
Q1
≤17.1
Vedo
W6
PBO
7%
SPYRE
6%
13%
17%
22%
37%
INDUCTION PK SIMULATIONS
SPY001 INDUCTION SIMULATION BASED ON 50-DAY HALF-LIFE
Concentration (µg/mL)
150
100
50
0
+20%
Higher anti-a437 mAb exposure may
lead to more rapid remission
Source: ¹Vedolizumab exposure-response data from Rosario, M., et. al. (2017); Vedolizumab FDA Clinical Pharmacology Review
0
SPY001 SC
VEDO IV (per label)
Quartile 4: 36 µg/mL
2
SPY001: ALL modeled patients above
vedo Q4 trough concentration
Weeks
4
6
16View entire presentation