Cannabis Concentrates
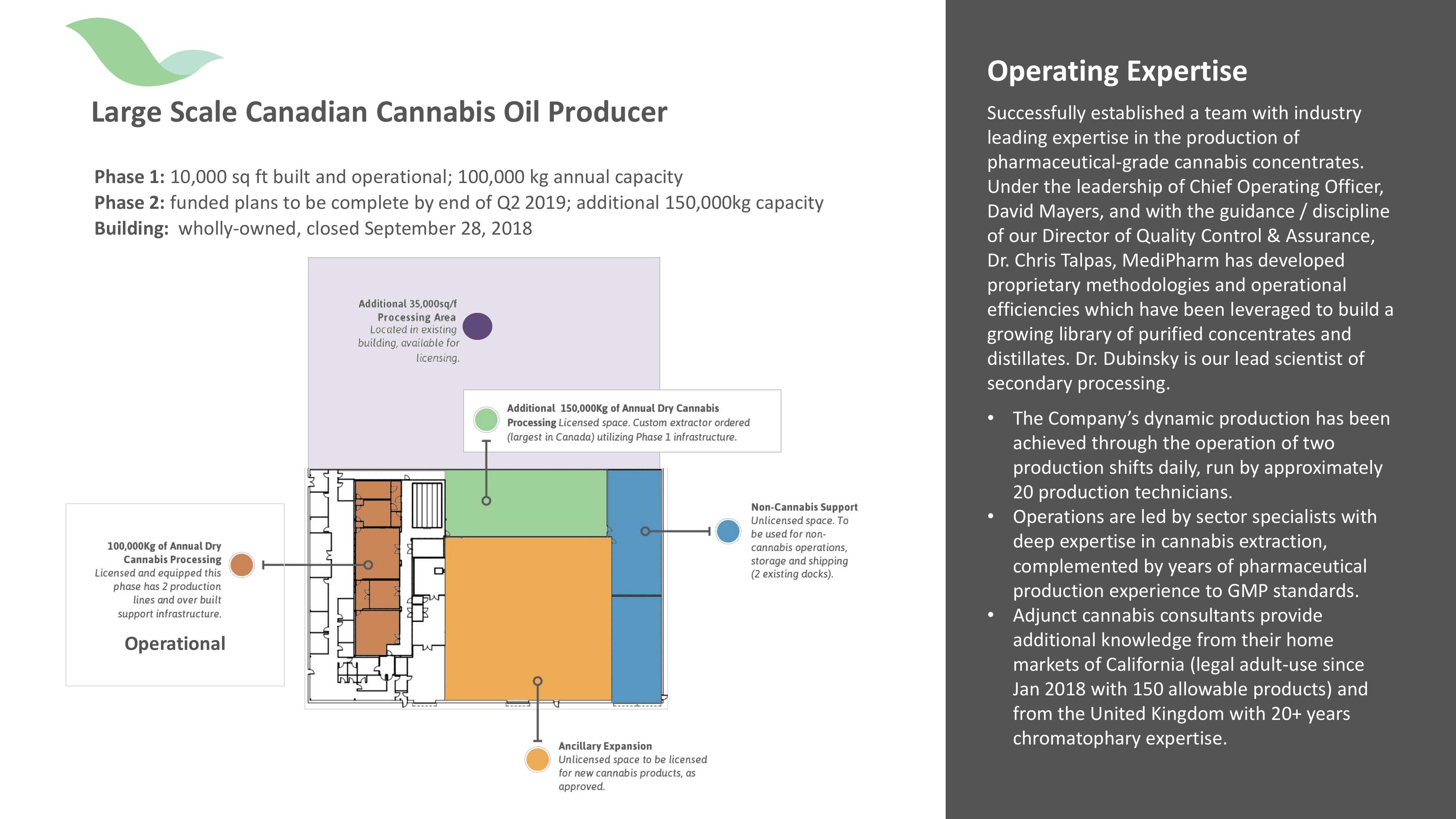
Large Scale Canadian Cannabis Oil Producer
Phase 1: 10,000 sq ft built and operational; 100,000 kg annual capacity
Phase 2: funded plans to be complete by end of Q2 2019; additional 150,000kg capacity
Building: wholly-owned, closed September 28, 2018
100,000kg of Annual Dry
Cannabis Processing
Licensed and equipped this
phase has 2 production
lines and over built
support infrastructure.
Operational
Additional 35,000sq/f
Processing Area
Located in existing
building, available for
licensing.
कल
Additional 150,000kg of Annual Dry Cannabis
Processing Licensed space. Custom extractor ordered
(largest in Canada) utilizing Phase 1 infrastructure.
Ancillary Expansion
Unlicensed space to be licensed
for new cannabis products, as
approved.
Non-Cannabis Support
Unlicensed space. To
be used for non-
cannabis operations,
storage and shipping
(2 existing docks).
Operating Expertise
Successfully established a team with industry
leading expertise in the production of
pharmaceutical-grade cannabis concentrates.
Under the leadership of Chief Operating Officer,
David Mayers, and with the guidance / discipline
of our Director of Quality Control & Assurance,
Dr. Chris Talpas, MediPharm has developed
proprietary methodologies and operational
efficiencies which have been leveraged to build a
growing library of purified concentrates and
distillates. Dr. Dubinsky is our lead scientist of
secondary processing.
The Company's dynamic production has been
achieved through the operation of two
production shifts daily, run by approximately
20 production technicians.
Operations are led by sector specialists with
deep expertise in cannabis extraction,
complemented by years of pharmaceutical
production experience to GMP standards.
Adjunct cannabis consultants provide
additional knowledge from their home
markets of California (legal adult-use since
Jan 2018 with 150 allowable products) and
from the United Kingdom with 20+ years
chromatophary expertise.View entire presentation