AbCellera Results Presentation Deck
FINANCIALS
Q2 2023 BUSINESS UPDATE
COPYRIGHT © ABCELLERA
9
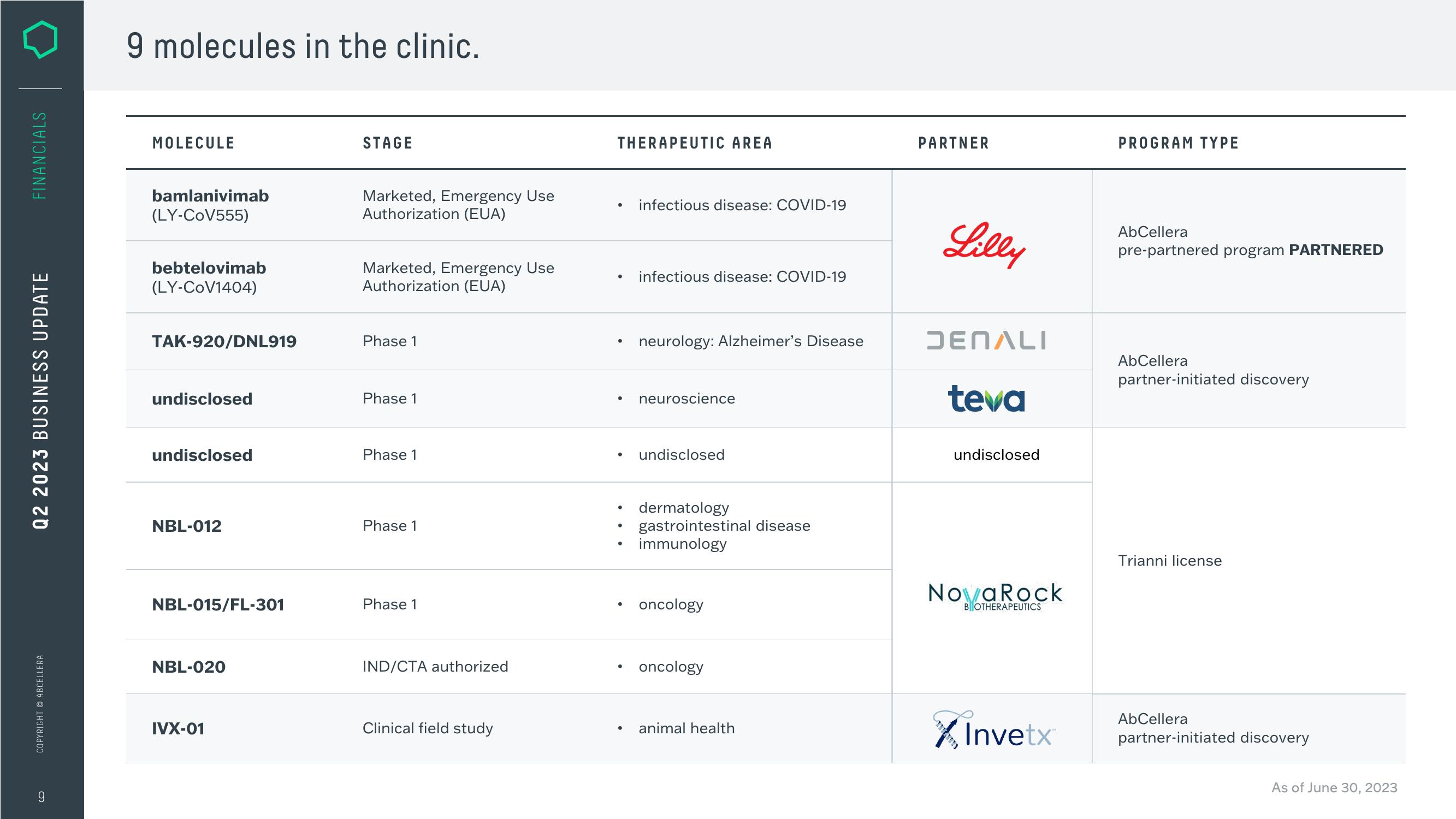
9 molecules in the clinic.
MOLECULE
bamlanivimab
(LY-CoV555)
bebtelovimab
(LY-CoV1404)
TAK-920/DNL919
undisclosed
undisclosed
NBL-012
NBL-015/FL-301
NBL-020
IVX-01
STAGE
Marketed, Emergency Use
Authorization (EUA)
Marketed, Emergency Use
Authorization (EUA)
Phase 1
Phase 1
Phase 1
Phase 1
Phase 1
IND/CTA authorized
Clinical field study
THERAPEUTIC AREA
●
●
●
infectious disease: COVID-19
●
infectious disease: COVID-19
neurology: Alzheimer's Disease
neuroscience
undisclosed
dermatology
gastrointestinal disease
immunology
• oncology
oncology
animal health
PARTNER
Lilly
DENALI
teva
undisclosed
NovaRock
BOTHERAPEUTICS
Invetx
PROGRAM TYPE
AbCellera
pre-partnered program PARTNERED
AbCellera
partner-initiated discovery
Trianni license
AbCellera
partner-initiated discovery
As of June 30, 2023View entire presentation