BioNTech Investor Day Presentation Deck
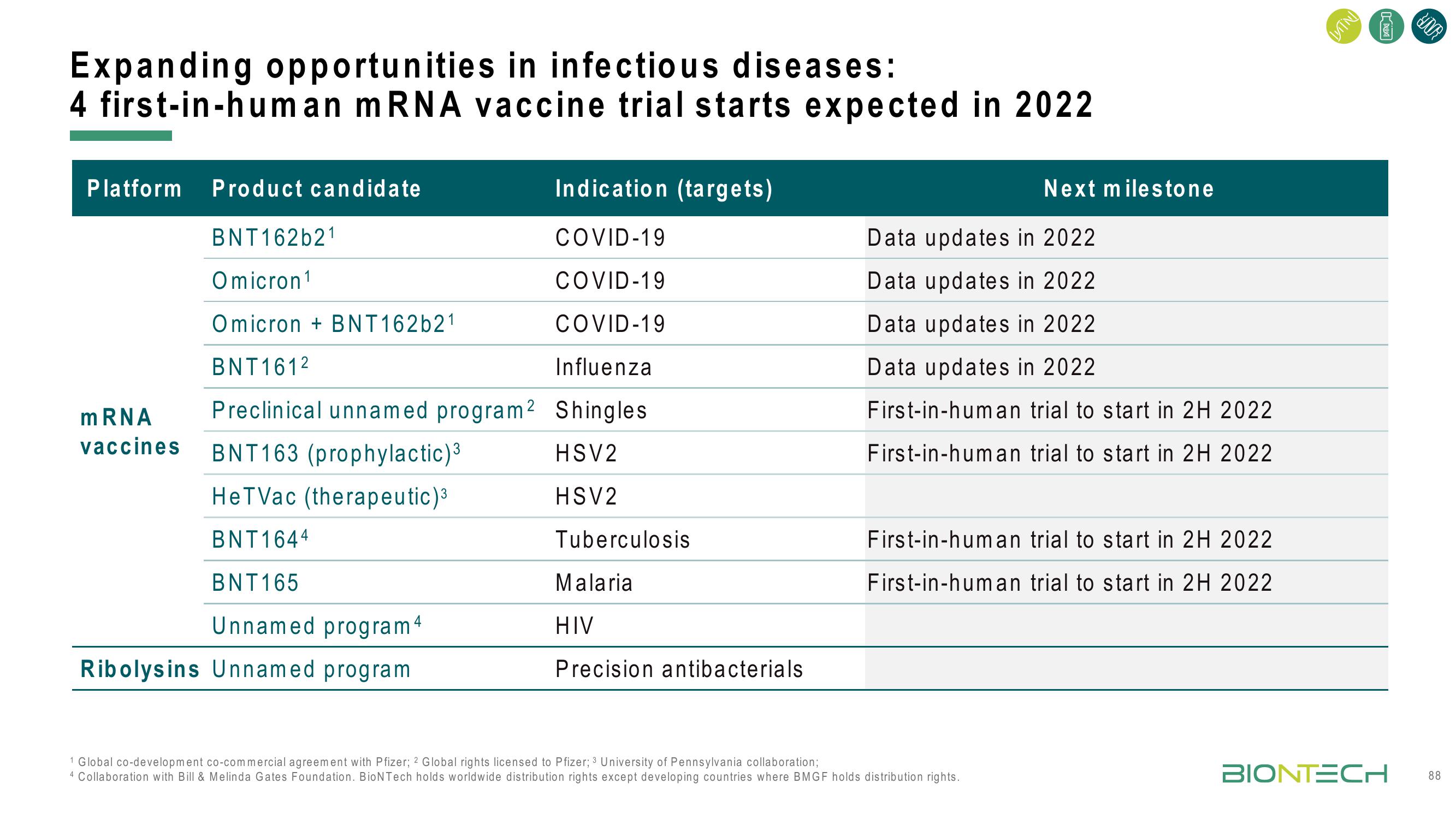
Expanding opportunities in infectious diseases:
4 first-in-human mRNA vaccine trial starts expected in 2022
Platform Product candidate
BNT162b21
Omicron ¹
Omicron + BNT162b21
BNT161²
Preclinical unnamed program²
BNT163 (prophylactic)³
HeTVac (therapeutic)³
BNT1644
mRNA
vaccines
BNT165
Unnamed program 4
Ribolysins Unnamed program
Indication (targets)
COVID-19
COVID-19
COVID-19
Influenza
Shingles
HSV2
HSV2
Tuberculosis
Malaria
HIV
Precision antibacterials
Next milestone
Data updates in 2022
Data updates in 2022
Data updates in 2022
Data updates in 2022
First-in-human trial to start in 2H 2022
First-in-human trial to start in 2H 2022
First-in-human trial to start in 2H 2022
First-in-human trial to start in 2H 2022
1 Global co-development co-commercial agreement with Pfizer; 2 Global rights licensed to Pfizer; 3 University of Pennsylvania collaboration;
4 Collaboration with Bill & Melinda Gates Foundation. BioNTech holds worldwide distribution rights except developing countries where BMGF holds distribution rights.
NUM
INUM
BIONTECH
YOUR
88View entire presentation