Ocuphire Pharma Results Presentation Deck
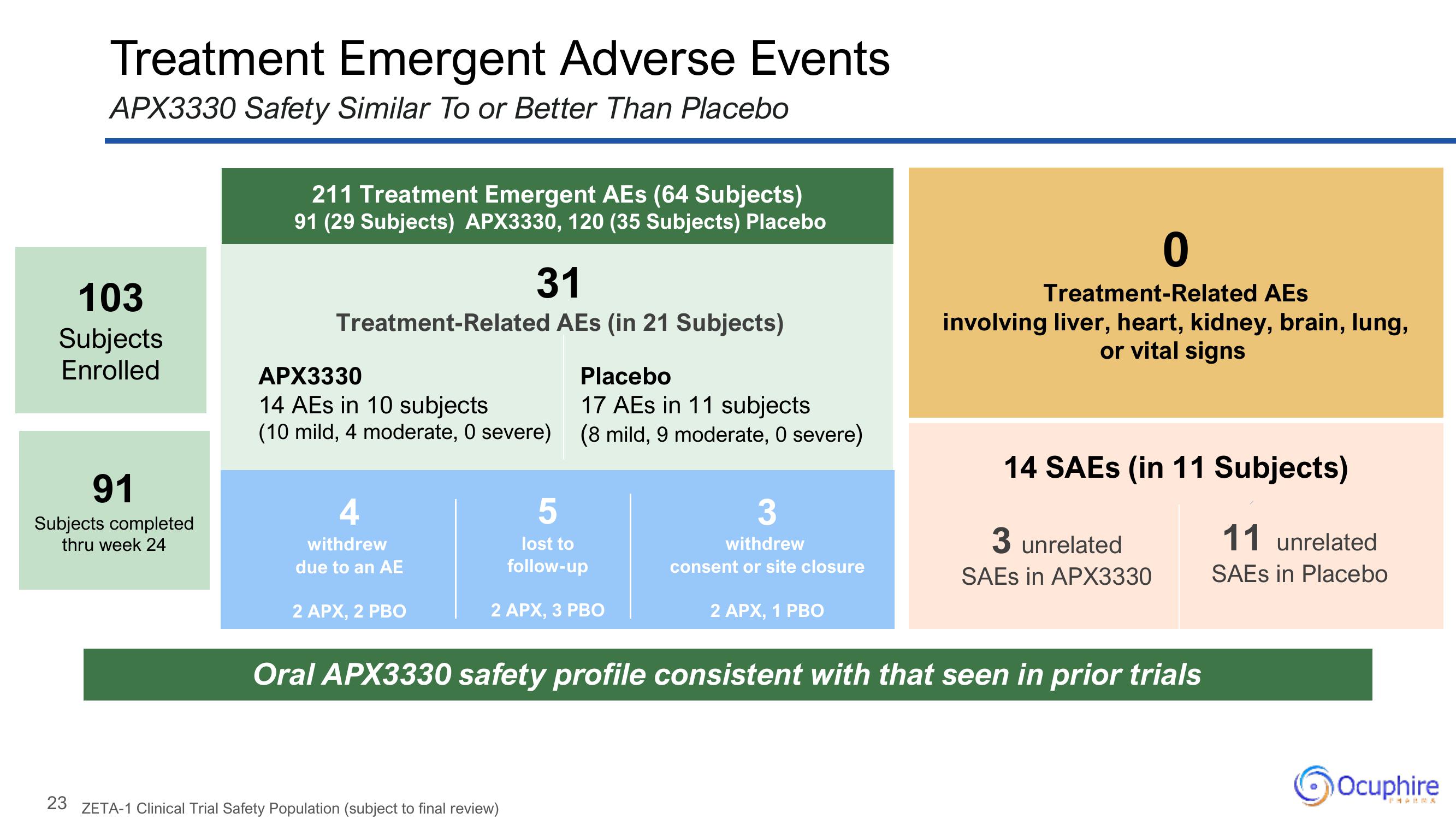
Treatment Emergent Adverse Events
APX3330 Safety Similar To or Better Than Placebo
103
Subjects
Enrolled
91
Subjects completed
thru week 24
211 Treatment Emergent AEs (64 Subjects)
91 (29 Subjects) APX3330, 120 (35 Subjects) Placebo
31
Treatment-Related AEs (in 21 Subjects)
APX3330
14 AEs in 10 subjects
(10 mild, 4 moderate, O severe)
withdrew
due to an AE
2 APX, 2 PBO
Placebo
17 AEs in 11 subjects
(8 mild, 9 moderate, 0 severe)
5
lost to
follow-up
2 APX, 3 PBO
23 ZETA-1 Clinical Trial Safety Population (subject to final review)
3
withdrew
consent or site closure
2 APX, 1 PBO
0
Treatment-Related AES
involving liver, heart, kidney, brain, lung,
or vital signs
14 SAEs (in 11 Subjects)
3 unrelated
SAEs in APX3330
Oral APX3330 safety profile consistent with that seen in prior trials
11 unrelated
SAES in Placebo
OcuphireView entire presentation