LumiraDx Investor Presentation Deck
3
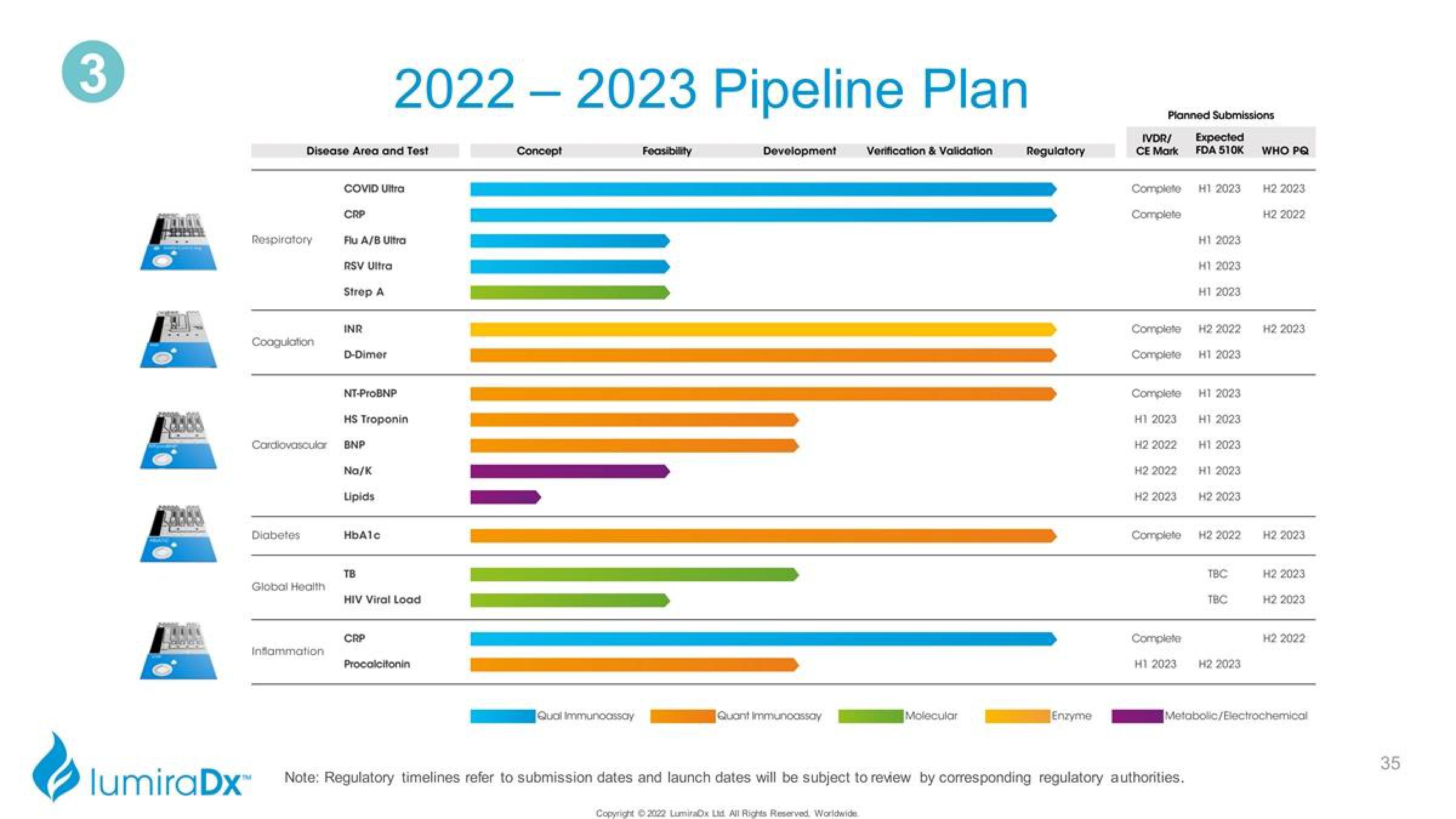
Respiratory
Coagulation
Disease Area and Test
Diabetes
Global Health
Inflammation
lumiraDx™
COVID Ultra
CRP
Flu A/B Ultra
RSV Ultra
Strep A
INR
Cardiovascular BNP
D-Dimer
NT-ProBNP
2022 2023 Pipeline Plan
HS Troponin
Na/K
Lipids
HbAlc
TB
HIV Viral Load
CRP
Focacifonin
Concept
Qual Immunoassay
Feasibility
Development
Quant Immunoassay
Verification & Validation
Copyright © 2022 LumiraDx Ltd. All Rights Reserved. Worldwide
Molecular
Regulatory
E
Enzyme
Planned Submissions
IVDR/
CE Mark
Complete
Complete
Complete
Complete
Complete
H1 2073
H2 2022
H2 2022
H2 2023
Complete
Complete
H1 2023
Note: Regulatory timelines refer to submission dates and launch dates will be subject to review by corresponding regulatory authorities.
Expected
FDA 510K
H1 223
H1 2023
H1123
HT 2023
H2 2022
H1 2023
HI 2023
H1 2023
HV 2023
Hi 2023
H2 2023
H2 2022
TBC
TBC
H2 2023
WHO PQ
H2 2023
H2 2022
H2 2023
H2 2023
H2 2023
H2 2023
H2 2022
Metabolic/Electrochemical
35View entire presentation