MaxCyte IPO Presentation Deck
Lentiviral
⠀
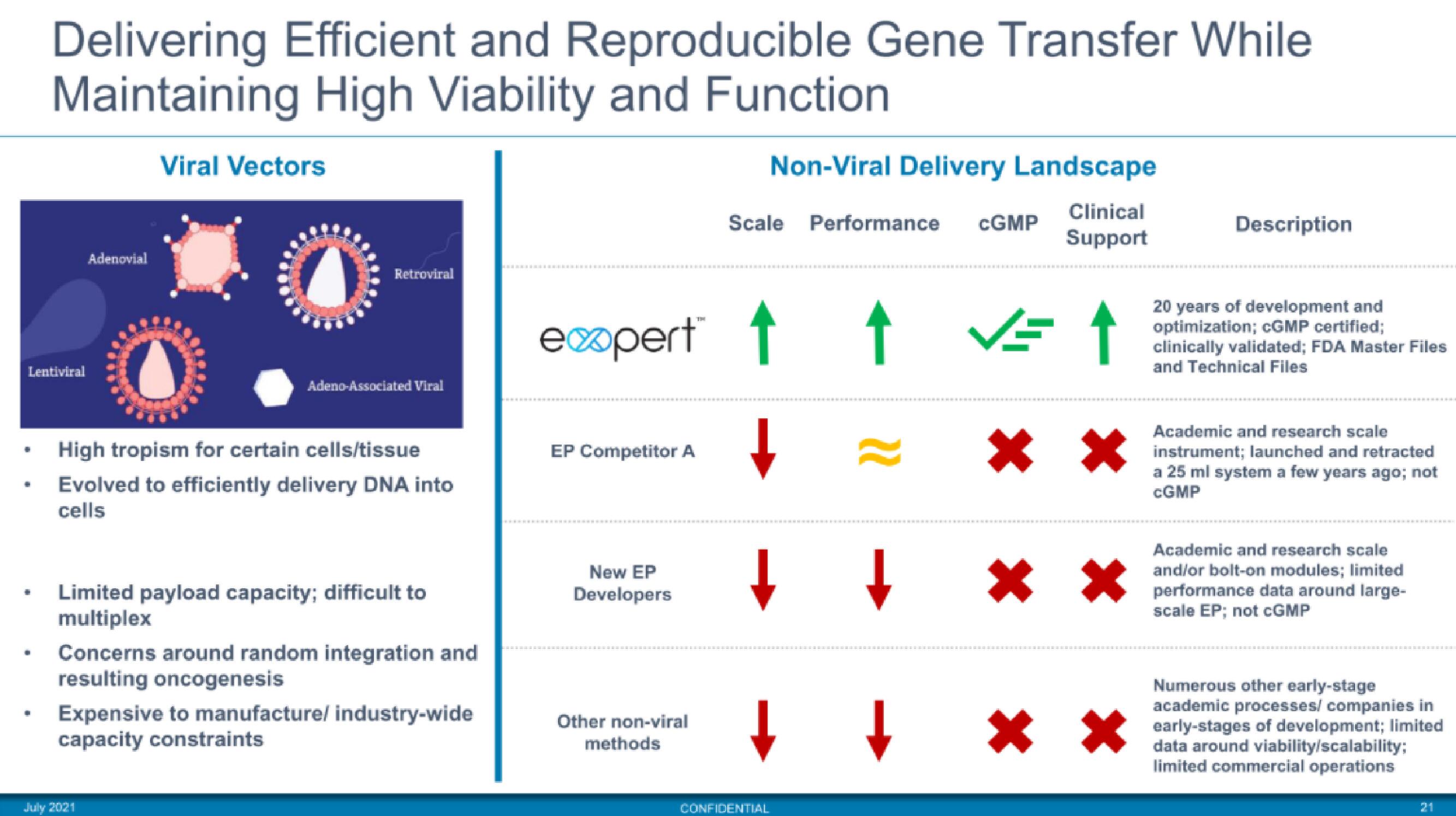
Delivering Efficient and Reproducible Gene Transfer While
Maintaining High Viability and Function
Viral Vectors
-
Adenovial
Retroviral
Adeno-Associated Viral
High tropism for certain cells/tissue
Evolved to efficiently delivery DNA into
cells
Limited payload capacity; difficult to
multiplex
July 2021
Concerns around random integration and
resulting oncogenesis
Expensive to manufacture/ industry-wide
capacity constraints
EP Competitor A
expert™ ↑
↑
New EP
Developers
Other non-viral
methods
Non-Viral Delivery Landscape
Clinical
Support
Scale Performance CGMP
↓
CONFIDENTIAL
↑
"
↓
✓= ↑
* *
* *
↓ ↓ * *
Description
20 years of development and
optimization; cGMP certified;
clinically validated; FDA Master Files
and Technical Files
Academic and research scale
instrument; launched and retracted
a 25 ml system a few years ago; not
CGMP
Academic and research scale
and/or bolt-on modules; limited
performance data around large-
scale EP; not cGMP
Numerous other early-stage
academic processes/ companies in
early-stages of development; limited
data around viability/scalability;
limited commercial operationsView entire presentation