Ocuphire Pharma Investor Presentation Deck
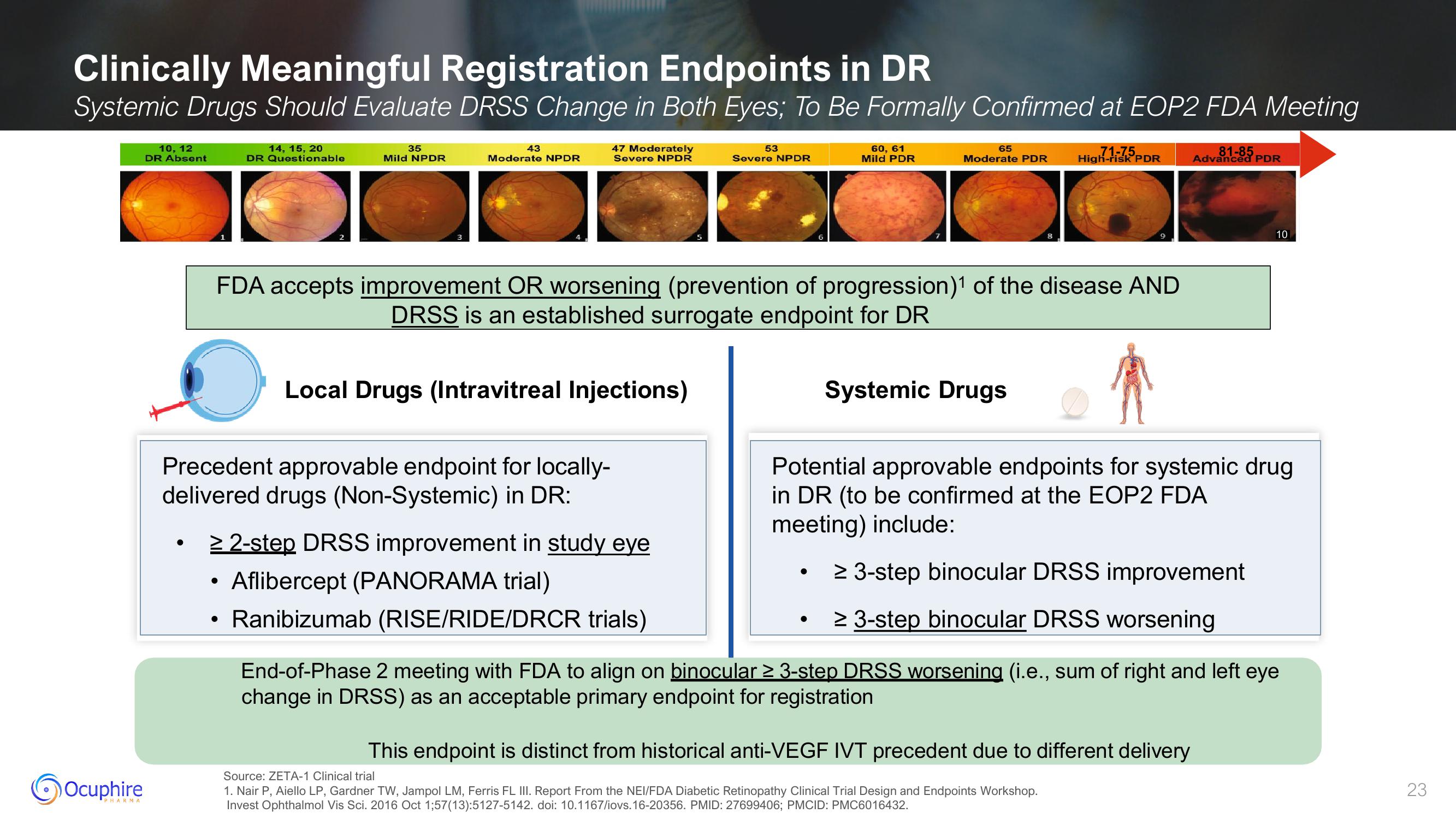
Clinically Meaningful Registration Endpoints in DR
Systemic Drugs Should Evaluate DRSS Change in Both Eyes; To Be Formally Confirmed at EOP2 FDA Meeting
Ocuphire
10, 12
DR Absent
14, 15, 20
DR Questionable
35
Mild NPDR
●
43
●
Moderate NPDR
Precedent approvable endpoint for locally-
delivered drugs (Non-Systemic) in DR:
47 Moderately
Severe NPDR
Local Drugs (Intravitreal Injections)
≥ 2-step DRSS improvement in study eye
Aflibercept (PANORAMA trial)
Ranibizumab (RISE/RIDE/DRCR trials)
FDA accepts improvement OR worsening (prevention of progression)¹ of the disease AND
DRSS is an established surrogate endpoint for DR
53
Severe NPDR
60, 61
Mild PDR
65
71-75
Moderate PDR High-risk PDR
●
Systemic Drugs
Potential approvable endpoints for systemic drug
in DR (to be confirmed at the EOP2 FDA
meeting) include:
81-85
Advanced PDR
≥ 3-step binocular DRSS improvement
≥ 3-step binocular DRSS worsening
This endpoint is distinct from historical anti-VEGF IVT precedent due to different delivery
10
End-of-Phase 2 meeting with FDA to align on binocular ≥ 3-step DRSS worsening (i.e., sum of right and left eye
change in DRSS) as an acceptable primary endpoint for registration
Source: ZETA-1 Clinical trial
1. Nair P, Aiello LP, Gardner TW, Jampol LM, Ferris FL III. Report From the NEI/FDA Diabetic Retinopathy Clinical Trial Design and Endpoints Workshop.
Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5127-5142. doi: 10.1167/iovs. 16-20356. PMID: 27699406; PMCID: PMC6016432.
23View entire presentation