AstraZeneca Results Presentation Deck
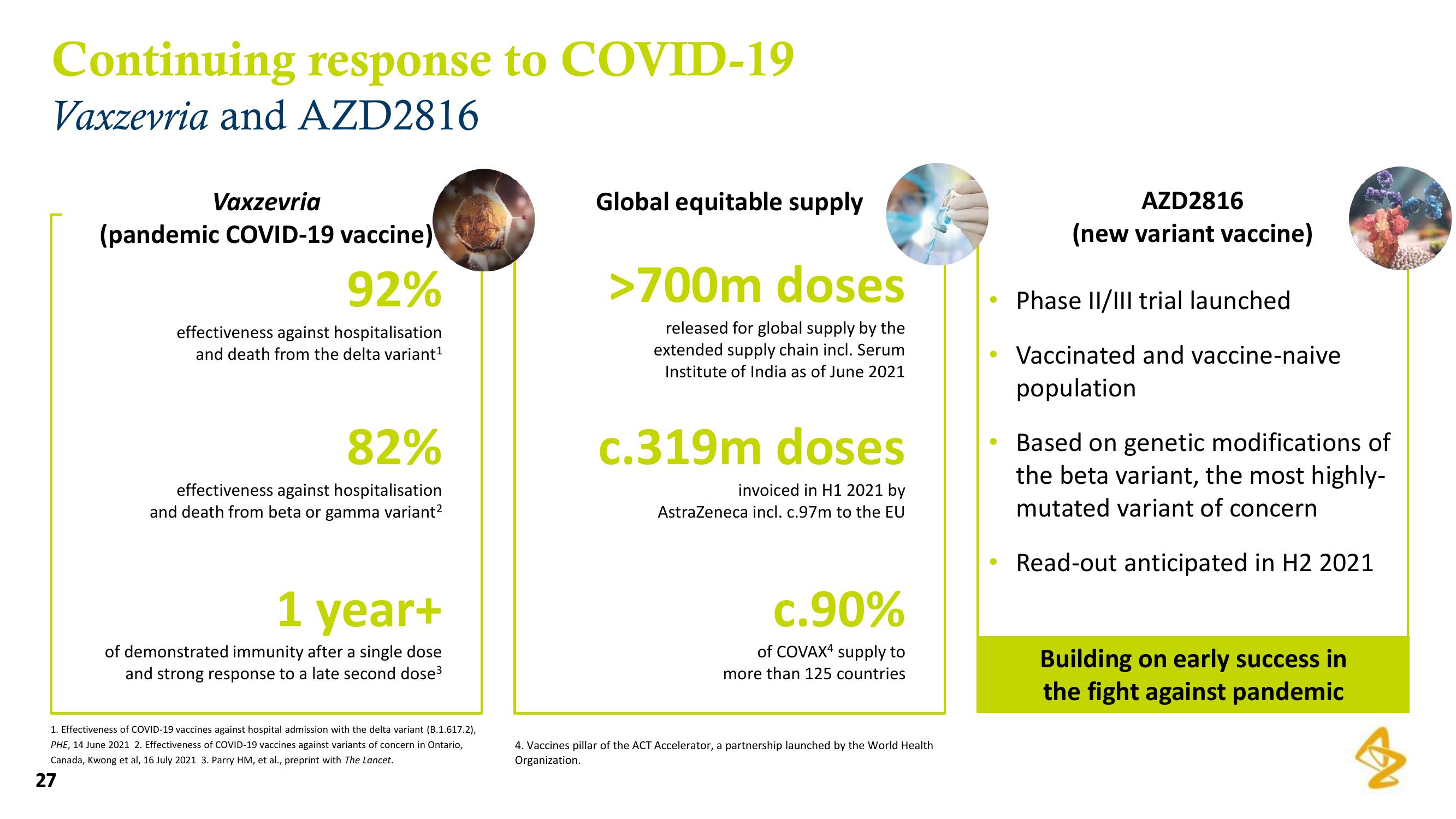
Continuing response to COVID-19
Vaxzevria and AZD2816
Vaxzevria
(pandemic COVID-19 vaccine)
92%
effectiveness against hospitalisation
and death from the delta variant¹
82%
effectiveness against hospitalisation
and death from beta or gamma variant²
1 year+
of demonstrated immunity after a single dose
and strong response to a late second dose³
1. Effectiveness of COVID-19 vaccines against hospital admission with the delta variant (B.1.617.2),
PHE, 14 June 2021 2. Effectiveness of COVID-19 vaccines against variants of concern in Ontario,
Canada, Kwong et al, 16 July 2021 3. Parry HM, et al., preprint with The Lancet.
27
Global equitable supply
>700m doses
released for global supply by the
extended supply chain incl. Serum
Institute of India as of June 2021
c.319m doses
invoiced in H1 2021 by
AstraZeneca incl. c.97m to the EU
c.90%
of COVAX4 supply to
more than 125 countries
4. Vaccines pillar of the ACT Accelerator, a partnership launched by the World Health
Organization.
AZD2816
(new variant vaccine)
Phase II/III trial launched
Vaccinated and vaccine-naive
population
Based on genetic modifications of
the beta variant, the most highly-
mutated variant of concern
Read-out anticipated in H2 2021
Building on early success in
the fight against pandemic
3View entire presentation