JP Morgan Healthcare Conference Innovation Update
VISION CARE INNOVATION
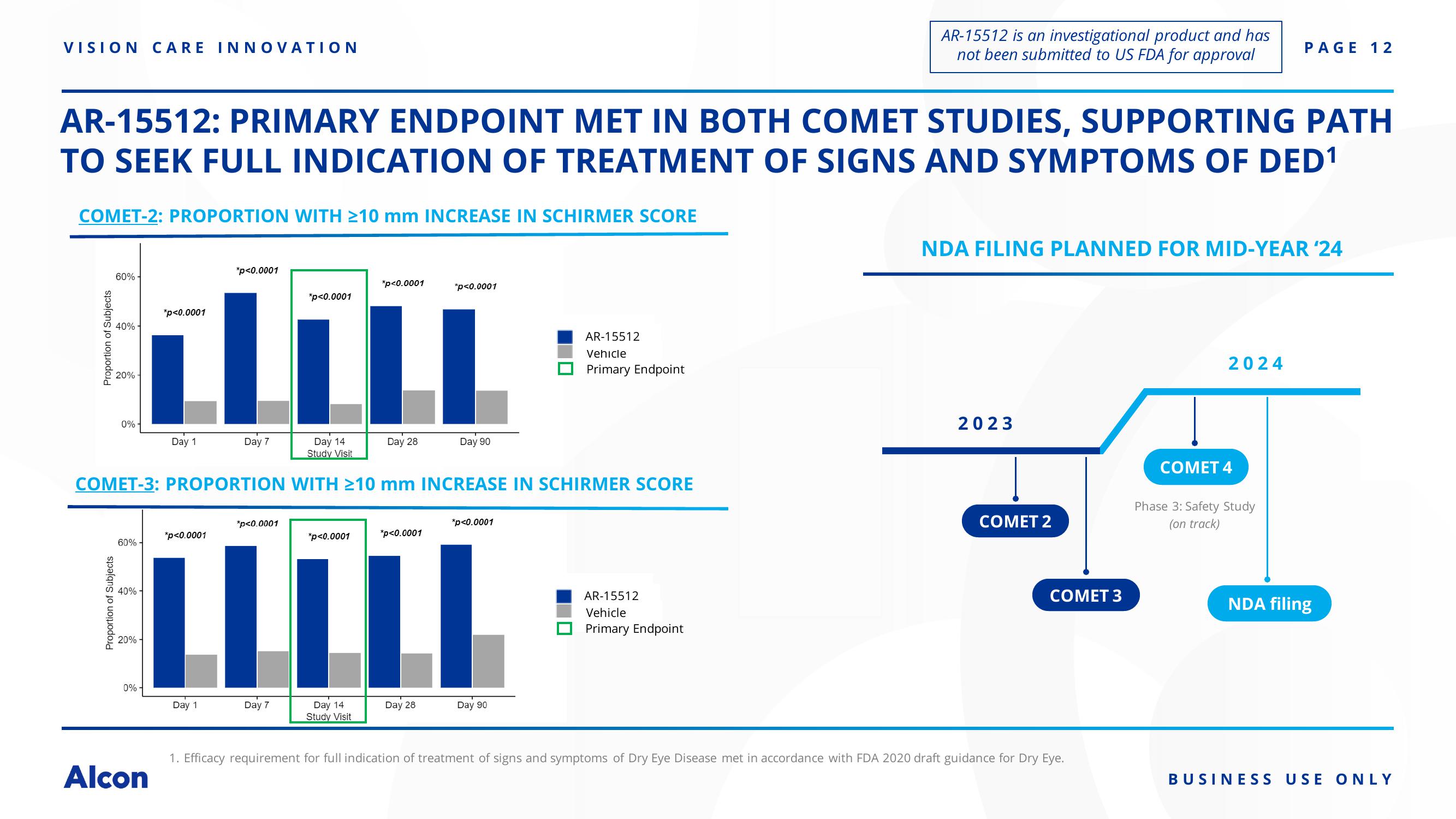
COMET-2: PROPORTION WITH ≥10 mm INCREASE IN SCHIRMER SCORE
AR-15512: PRIMARY ENDPOINT MET IN BOTH COMET STUDIES, SUPPORTING PATH
TO SEEK FULL INDICATION OF TREATMENT OF SIGNS AND SYMPTOMS OF DED¹
Proportion of Subjects
60%-
40%-
20%-
*p<0.0001
ilill
LLL
Day 28
0%-
60%-
40%-
20%
0%
*p<0.0001
Day 1
Alcon
*p<0.0001
Day 14
Study Visit
COMET-3: PROPORTION WITH ≥10 mm INCREASE IN SCHIRMER SCORE
*p<0.0001
Day 7
Day 1
*p<0.0001
*p<0.0001
*p<0.0001
LLLLL
Day 7
Day 14
Study Visit
*p<0.0001
*p<0.0001
Day 90
Day 28
*p<0.0001
AR-15512
Vehicle
Primary Endpoint
Day 90
AR-15512 is an investigational product and has
not been submitted to US FDA for approval
AR-15512
Vehicle
Primary Endpoint
NDA FILING PLANNED FOR MID-YEAR '24
2023
COMET 2
COMET 3
1. Efficacy requirement for full indication of treatment of signs and symptoms of Dry Eye Disease met in accordance with FDA 2020 draft guidance for Dry Eye.
2024
PAGE 12
COMET 4
Phase 3: Safety Study
(on track)
NDA filing
BUSINESS USE ONLYView entire presentation