Kymera Investor Presentation Deck
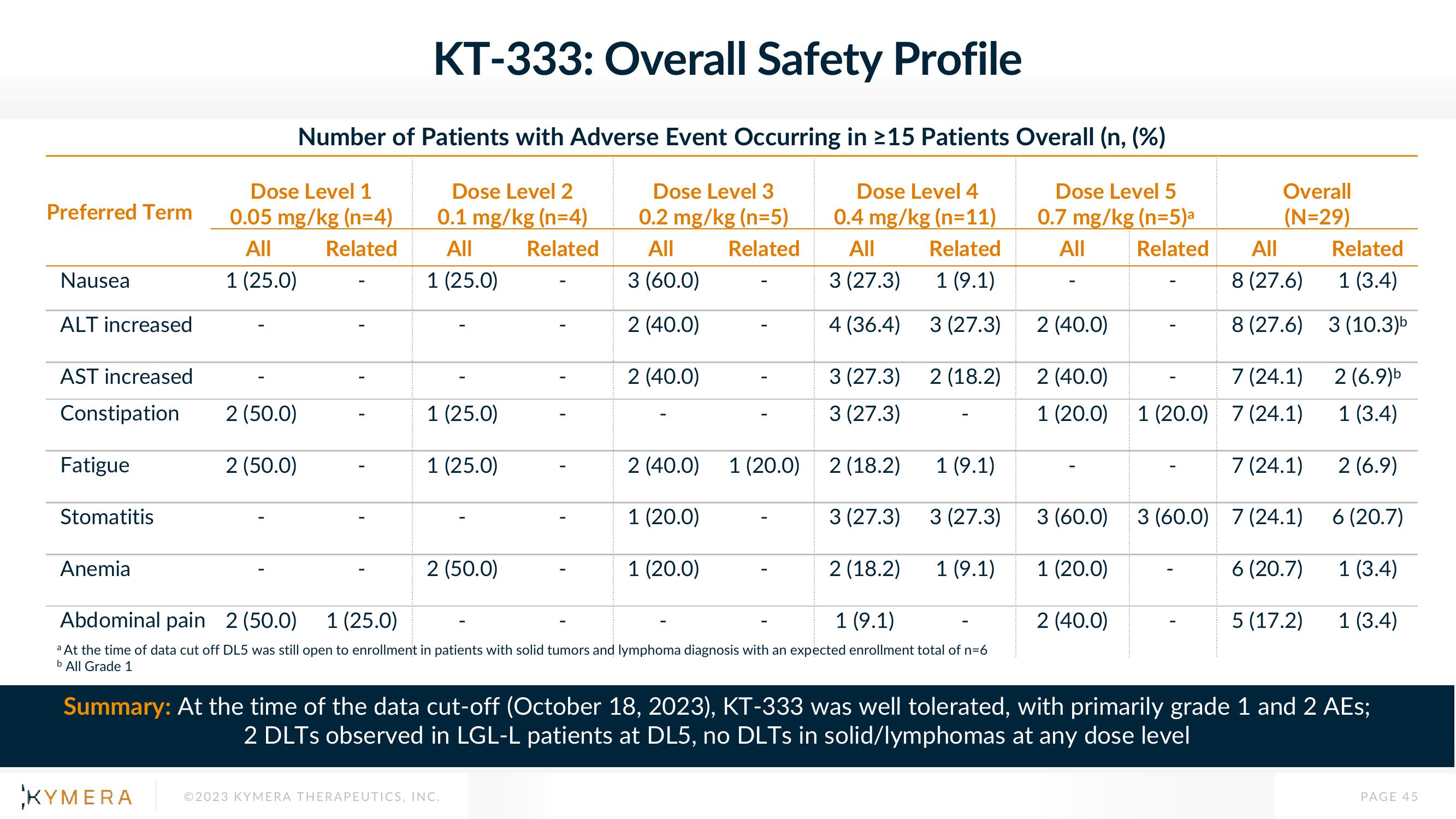
Preferred Term
Nausea
ALT increased
AST increased
Constipation
Fatigue
Stomatitis
Anemia
Number of Patients with Adverse Event Occurring in ≥15 Patients Overall (n, (%)
Dose Level 1
0.05 mg/kg (n=4)
Dose Level 2
0.1 mg/kg (n=4)
All Related
Dose Level 3
0.2 mg/kg (n=5)
All Related
Dose Level 4
0.4 mg/kg (n=11)
All Related
3 (27.3) 1 (9.1)
Dose Level 5
0.7 mg/kg (n=5)a
All Related
Related
All
1 (25.0)
1 (25.0)
3 (60.0)
2 (40.0)
4 (36.4)
3 (27.3)
2 (50.0)
KT-333: Overall Safety Profile
2 (50.0)
1 (25.0)
1 (25.0)
2 (50.0)
2 (40.0)
2 (40.0)
1 (20.0)
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
1 (20.0)
1 (20.0)
3 (27.3)
3 (27.3)
2 (18.2)
3 (27.3)
2 (18.2)
2 (18.2)
1 (9.1)
3 (27.3)
1 (9.1)
1 (9.1)
Abdominal pain 2 (50.0) 1 (25.0)
a At the time of data cut off DL5 was still open to enrollment in patients with solid tumors and lymphoma diagnosis with an expected enrollment total of n=6
b All Grade 1
2 (40.0)
2 (40.0)
1 (20.0)
3 (60.0)
1 (20.0)
2 (40.0)
Overall
(N=29)
All
8 (27.6)
8 (27.6)
7 (24.1)
1 (20.0) 7 (24.1)
7 (24.1)
3 (60.0) 7 (24.1)
6 (20.7)
5 (17.2)
Related
1 (3.4)
3 (10.3)b
2 (6.9)b
1 (3.4)
2 (6.9)
6 (20.7)
1 (3.4)
1 (3.4)
Summary: At the time of the data cut-off (October 18, 2023), KT-333 was well tolerated, with primarily grade 1 and 2 AEs;
2 DLTs observed in LGL-L patients at DL5, no DLTs in solid/lymphomas at any dose level
PAGE 45View entire presentation