BioAtla Investor Presentation Deck
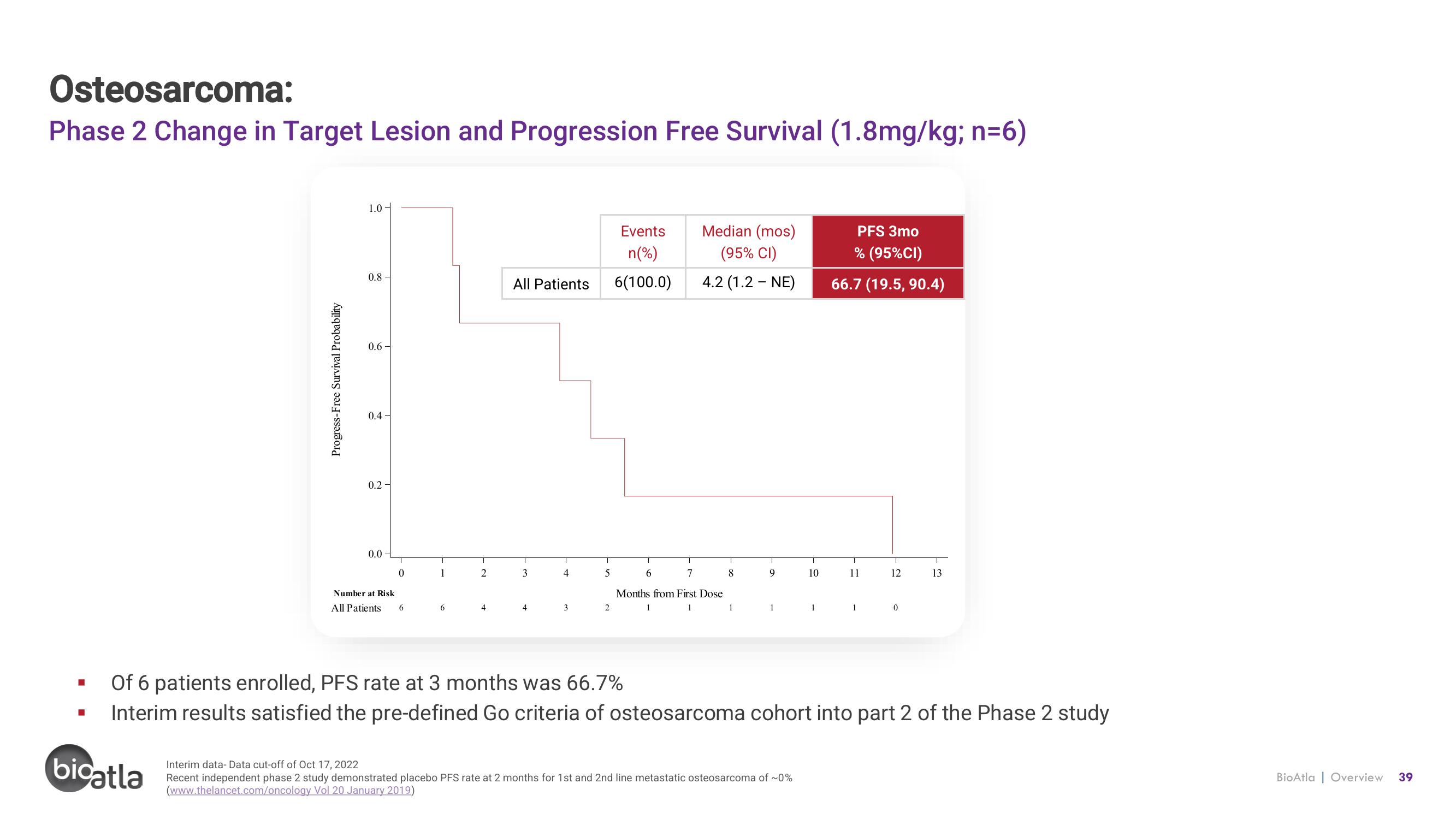
Osteosarcoma:
Phase 2 Change in Target Lesion and Progression Free Survival (1.8mg/kg; n=6)
■
Progress-Free Survival Probability
bicatla
1.0-
0.8
0.6
0.4
0.2
0.0
0
Number at Risk
All Patients 6
1
6
T
2
4
All Patients
3
4
T
4
3
T
2
Events
n(%)
6(100.0)
6
T
7
Median (mos)
(95% CI)
4.2 (1.2 - NE)
Months from First Dose
1
1
8
1
T
9
1
T
10
Interim data- Data cut-off of Oct 17, 2022
Recent independent phase 2 study demonstrated placebo PFS rate at 2 months for 1st and 2nd line metastatic osteosarcoma of ~0%
(www.thelancet.com/oncology Vol 20 January 2019)
1
PFS 3mo
% (95% CI)
66.7 (19.5, 90.4)
T
11
12
0
Of 6 patients enrolled, PFS rate at 3 months was 66.7%
Interim results satisfied the pre-defined Go criteria of osteosarcoma cohort into part 2 of the Phase 2 study
T
13
BioAtla| Overview
39View entire presentation