Bausch+Lomb Results Presentation Deck
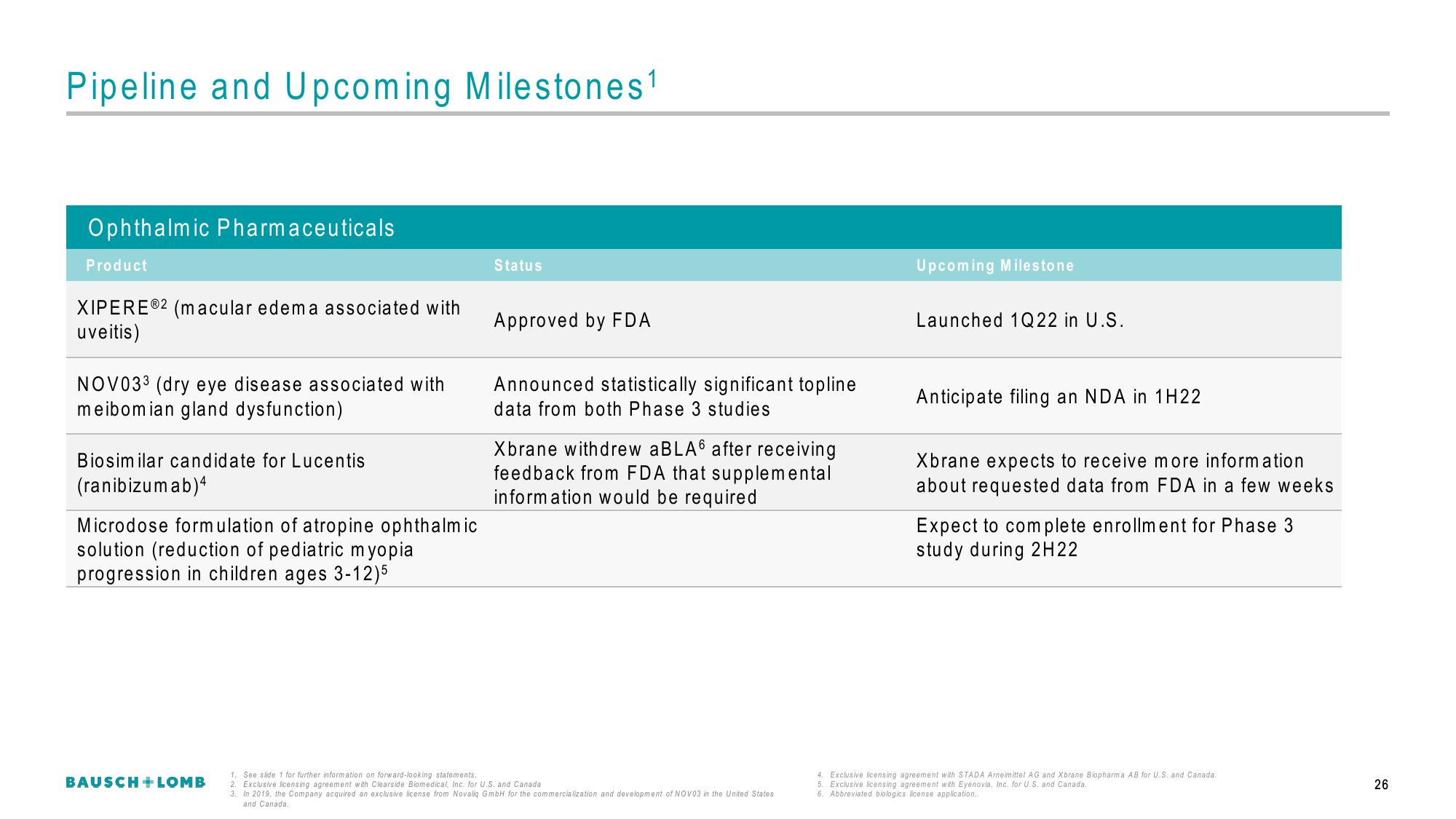
Pipeline and Upcoming Milestones¹
Ophthalmic Pharmaceuticals
Product
XIPEREⓇ2 (macular edema associated with
uveitis)
NOV033 (dry eye disease associated with
meibomian gland dysfunction)
Biosimilar candidate for Lucentis
(ranibizumab) 4
Microdose formulation of atropine ophthalmic
solution (reduction of pediatric myopia
progression in children ages 3-12)5
BAUSCH + LOMB
Status
Approved by FDA
Announced statistically significant topline
data from both Phase 3 studies
Xbrane withdrew aBLA after receiving
feedback from FDA that supplemental
information would be required
See slide 1 for further information on forward-looking statements.
2. Exclusive licensing agreement with Clearside Biomedical, Inc. for U.S. and Canada
In 2019, the Company acquired an exclusive license from Novaliq GmbH for the commercialization and development of NOV03 in the United States
and Canada.
Upcoming Milestone
Launched 1Q22 in U.S.
Anticipate filing an NDA in 1H22
Xbrane expects to receive more information
about requested data from FDA in a few weeks
Expect to complete enrollment for Phase 3
study during 2H22
4. Exclusive licensing agreement with STADA Arneimittel AG and Xbrane Biopharma AB for U.S. and Canada.
5. Exclusive licensing agreement with Eyenovia, Inc. for U.S. and Canada.
6. Abbreviated biologics license application..
26View entire presentation