BioNTech Investor Day Presentation Deck
●
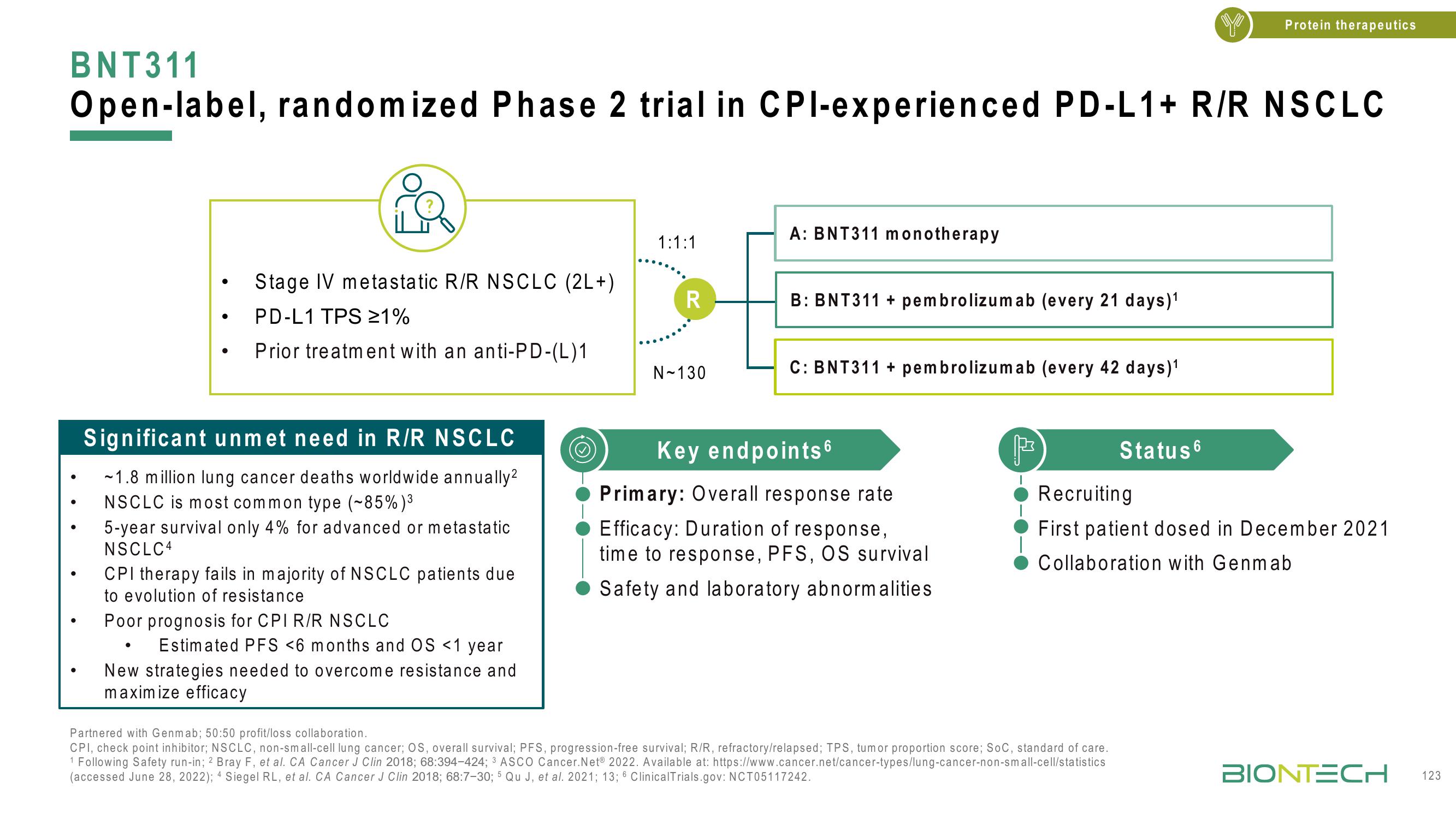
BNT311
Open-label, randomized Phase 2 trial in CPl-experienced PD-L1+ R/R NSCLC
Stage IV metastatic R/R NSCLC (2L+)
PD-L1 TPS ≥1%
Prior treatment with an anti-PD-(L)1
Significant unmet need in R/R NSCLC
~1.8 million lung cancer deaths worldwide annually²
NSCLC is most common type (~85%)³
5-year survival only 4% for advanced or metastatic
NSCLC4
CPI therapy fails in majority of NSCLC patients due
to evolution of resistance
Poor prognosis for CPI R/R NSCLC
Estimated PFS <6 months and OS <1 year
New strategies needed to overcome resistance and
maximize efficacy
1:1:1
R
N-130
A: BNT311 monotherapy
B: BNT311+ pembrolizumab (every 21 days)¹
C: BNT311+ pembrolizumab (every 42 days)¹
Key endpoints 6
Primary: Overall response rate
Efficacy: Duration of response,
time to response, PFS, OS survival
Safety and laboratory abnormalities
123
Y
Status 6
Partnered with Genmab; 50:50 profit/loss collaboration.
CPI, check point inhibitor; NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival; R/R, refractory/relapsed; TPS, tumor proportion score; SoC, standard of care.
1 Following Safety run-in; 2 Bray F, et al. CA Cancer J Clin 2018; 68:394-424; 3 ASCO Cancer.Net® 2022. Available at: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics
(accessed June 28, 2022); 4 Siegel RL, et al. CA Cancer J Clin 2018; 68:7-30; 5 Qu J, et al. 2021; 13; 6 Clinical Trials.gov: NCT05117242.
Protein therapeutics
Recruiting
First patient dosed in December 2021
Collaboration with Genmab
BIONTECH
123View entire presentation