Kymera Investor Day Presentation Deck
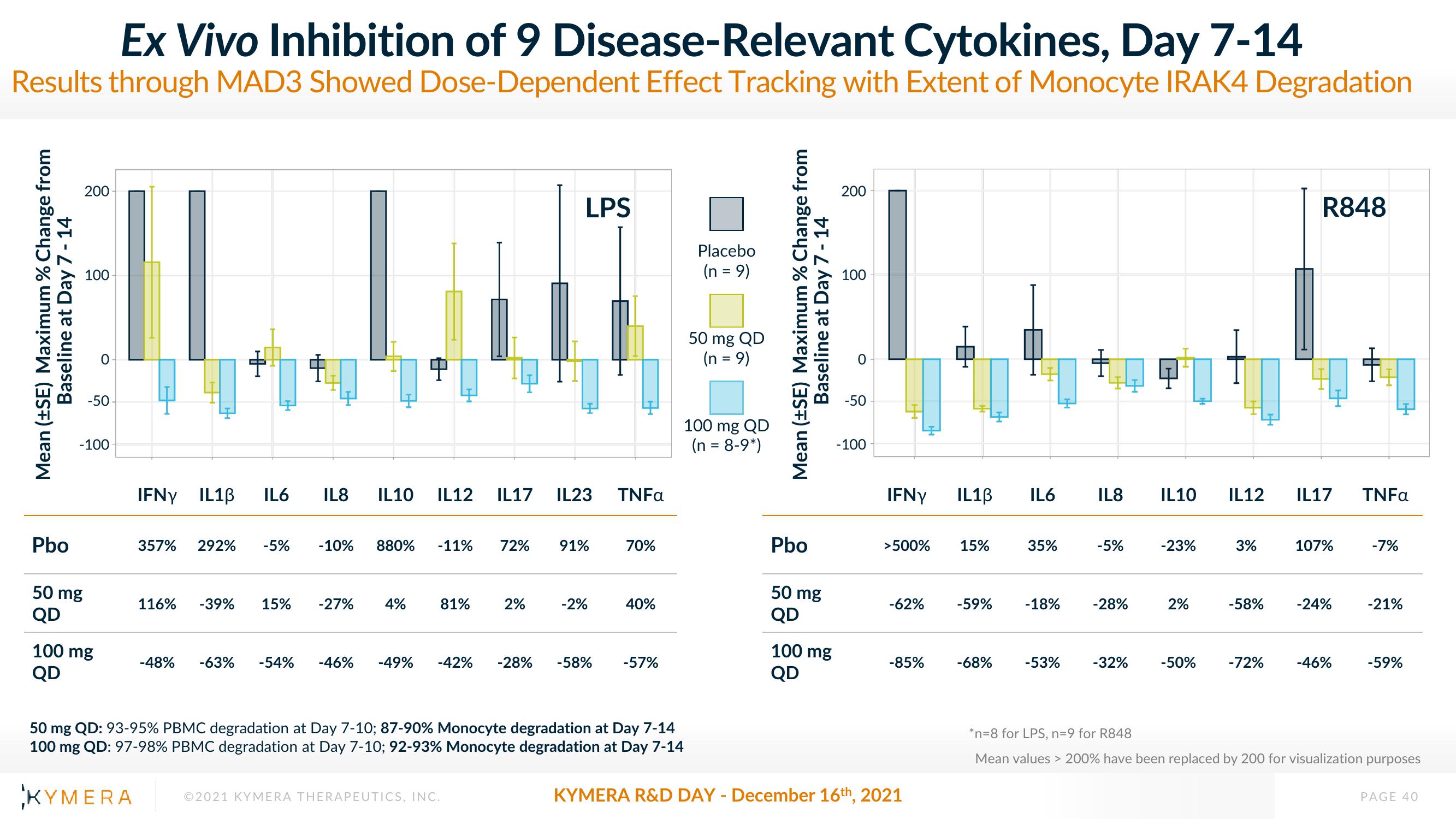
Ex Vivo Inhibition of 9 Disease-Relevant Cytokines, Day 7-14
Results through MAD3 Showed Dose-Dependent Effect Tracking with Extent of Monocyte IRAK4 Degradation
Pbo
200
50 mg
QD
100
-50
-100
100 mg
QD
IFNY IL10 IL6 IL8
116% -39% 15%
IL10 IL12 IL17 IL23
357% 292% -5% -10% 880% -11% 72% 91%
LPS
-27% 4%
81% 2%
-2%
-48% -63% -54% -46% -49% -42% -28% -58%
TNFa
70%
40%
-57%
50 mg QD: 93-95% PBMC degradation at Day 7-10; 87-90% Monocyte degradation at Day 7-14
100 mg QD: 97-98% PBMC degradation at Day 7-10; 92-93% Monocyte degradation at Day 7-14
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
Placebo
(n = 9)
50 mg QD
(n = 9)
100 mg QD
(n = 8-9*)
Pbo
50 mg
QD
100 mg
QD
200
100
0
-50
-100
IFNY
>500%
-62%
-85%
KYMERA R&D DAY - December 16th, 2021
H
IL1B
15%
-59%
-68%
IL6
35%
-18%
-53%
+4
IL8
-5%
-28%
-32%
IL10
-23%
2%
-50%
IL12
3%
-58%
-72%
R848
IL17
107%
-24%
-46%
TNFa
-7%
-21%
-59%
*n=8 for LPS, n=9 for R848
Mean values > 200% have been replaced by 200 for visualization purposes
PAGE 40View entire presentation