Kymera Investor Day Presentation Deck
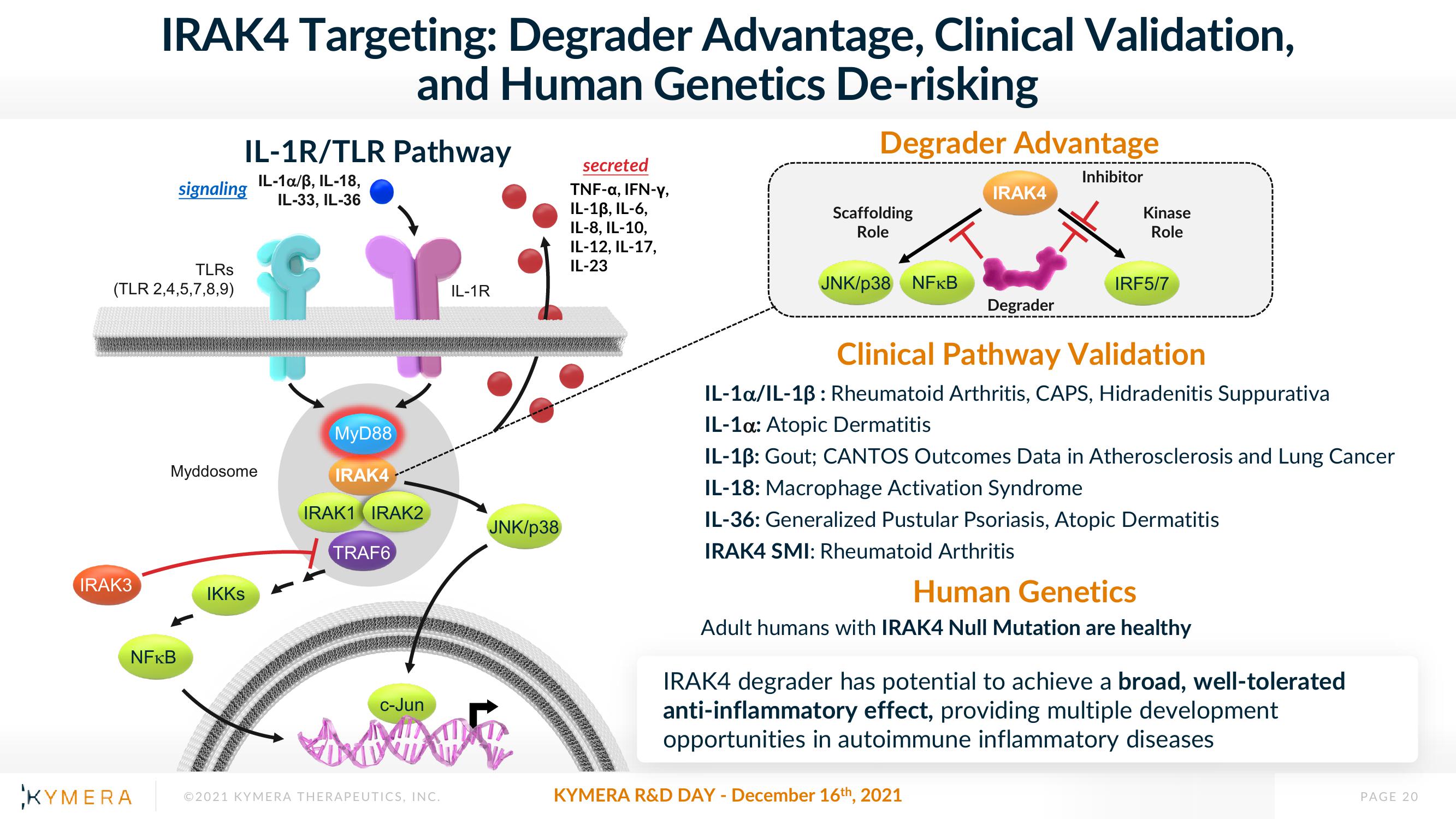
IRAK4 Targeting: Degrader Advantage, Clinical Validation,
and Human Genetics De-risking
Degrader Advantage
Inhibitor
IRAK3
TLRs
(TLR 2,4,5,7,8,9)
signaling
NFkB
IL-1R/TLR Pathway
IL-1 a/B, IL-18,
IL-33, IL-36
$
Myddosome
IKKS
MyD88
IRAK4
IRAK1 IRAK2
TRAF6
c-Jun
IL-1R
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
JNK/p38
MINITAT
secreted
TNF-a, IFN-y,
IL-1B, IL-6,
IL-8, IL-10,
IL-12, IL-17,
IL-23
Scaffolding
Role
JNK/p38 NFkB
IRAK4
Degrader
Kinase
Role
KYMERA R&D DAY - December 16th, 2021
IRF5/7
Clinical Pathway Validation
IL-1a/IL-13: Rheumatoid Arthritis, CAPS, Hidradenitis Suppurativa
IL-1α: Atopic Dermatitis
IL-1ß: Gout; CANTOS Outcomes Data in Atherosclerosis and Lung Cancer
IL-18: Macrophage Activation Syndrome
IL-36: Generalized Pustular Psoriasis, Atopic Dermatitis
IRAK4 SMI: Rheumatoid Arthritis
Human Genetics
Adult humans with IRAK4 Null Mutation are healthy
IRAK4 degrader has potential to achieve a broad, well-tolerated
anti-inflammatory effect, providing multiple development
opportunities in autoimmune inflammatory diseases
PAGE 20View entire presentation