BioAtla Investor Conference Presentation Deck
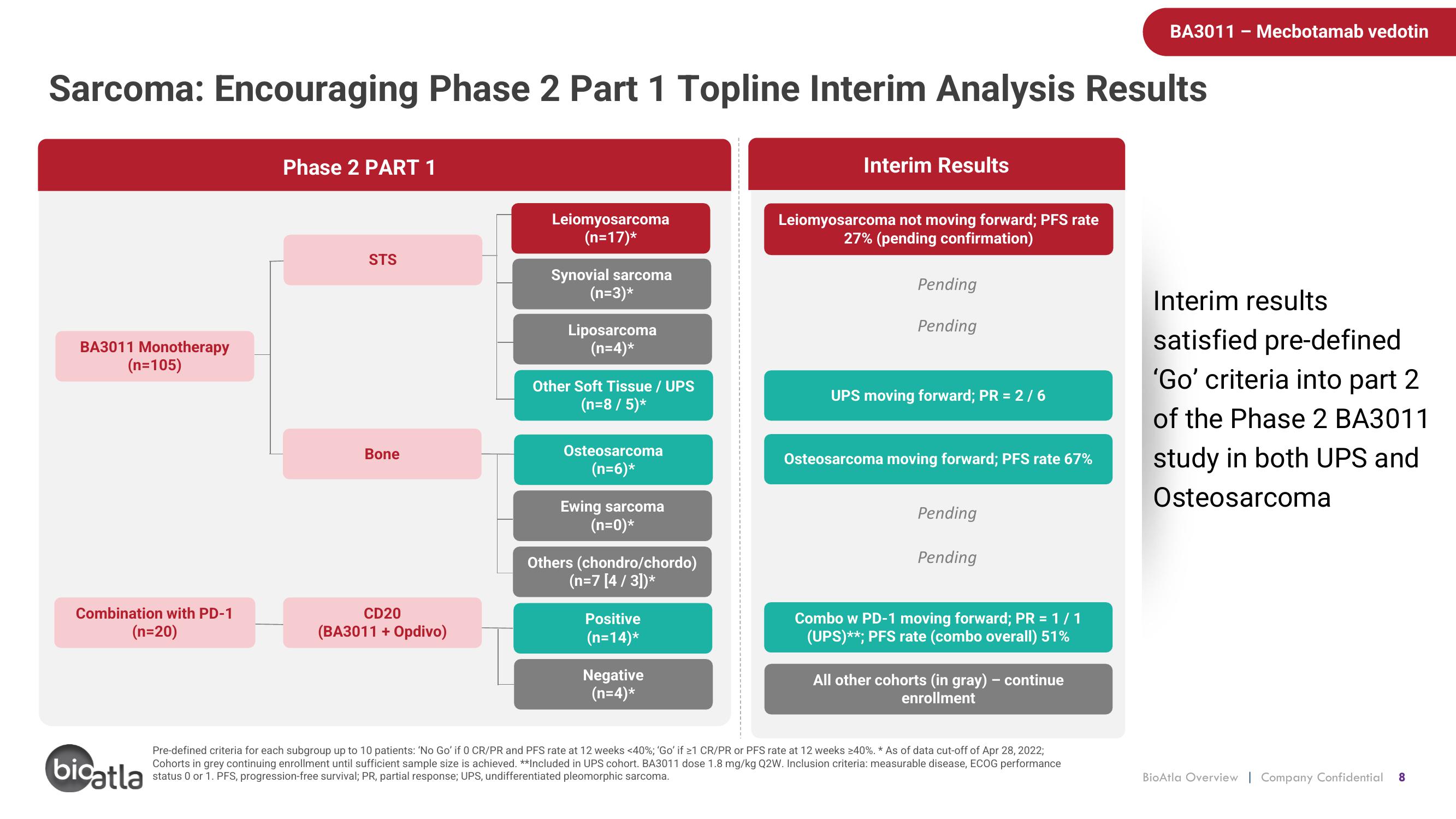
Sarcoma: Encouraging Phase 2 Part 1 Topline Interim Analysis Results
BA3011 Monotherapy
(n=105)
Combination with PD-1
(n=20)
bicatla
Phase 2 PART 1
STS
Bone
CD20
(BA3011 + Opdivo)
Leiomyosarcoma
(n=17)*
Synovial sarcoma
(n=3)*
Liposarcoma
(n=4)*
Other Soft Tissue / UPS
(n=8 / 5)*
Osteosarcoma
(n=6)*
Ewing sarcoma
(n=0)*
Others (chondro/chordo)
(n=7 [4 / 3])*
Positive
(n=14)*
Negative
(n=4)*
Interim Results
Leiomyosarcoma not moving forward; PFS rate
27% (pending confirmation)
Pending
Pending
UPS moving forward; PR = 2/6
Osteosarcoma moving forward; PFS rate 67%
Pending
Pending
Combo w PD-1 moving forward; PR = 1/1
(UPS)**; PFS rate (combo overall) 51%
All other cohorts (in gray) – continue
enrollment
BA3011 - Mecbotamab vedotin
Pre-defined criteria for each subgroup up to 10 patients: 'No Go' if 0 CR/PR and PFS rate at 12 weeks <40%; 'Go' if ≥1 CR/PR or PFS rate at 12 weeks >40%. * As of data cut-off of Apr 28, 2022;
Cohorts in grey continuing enrollment until sufficient sample size is achieved. **Included in UPS cohort. BA3011 dose 1.8 mg/kg Q2W. Inclusion criteria: measurable disease, ECOG performance
status 0 or 1. PFS, progression-free survival; PR, partial response; UPS, undifferentiated pleomorphic sarcoma.
Interim results
satisfied pre-defined
'Go' criteria into part 2
of the Phase 2 BA3011
study in both UPS and
Osteosarcoma
BioAtla Overview | Company Confidential
8View entire presentation