Kymera Results Presentation Deck
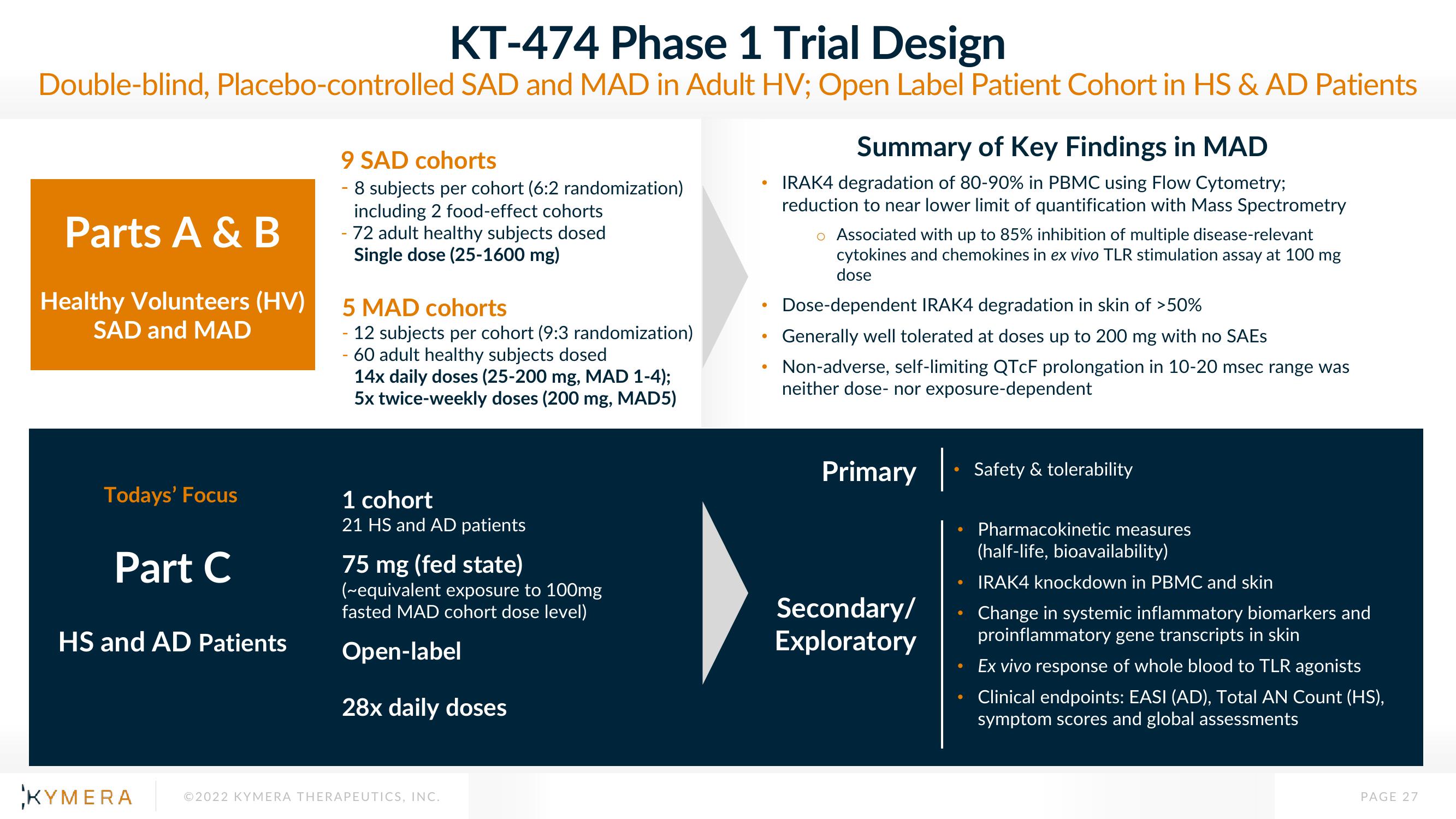
KT-474 Phase 1 Trial Design
Double-blind, placebo-controlled SAD and MAD in Adult HV; Open Label Patient Cohort in HS & AD Patients
Parts A & B
Healthy Volunteers (HV)
SAD and MAD
Todays' Focus
Part C
HS and AD Patients
9 SAD cohorts
- 8 subjects per cohort (6:2 randomization)
including 2 food-effect cohorts
- 72 adult healthy subjects dosed
Single dose (25-1600 mg)
5 MAD cohorts
- 12 subjects per cohort (9:3 randomization)
60 adult healthy subjects dosed
14x daily doses (25-200 mg, MAD 1-4);
5x twice-weekly doses (200 mg, MAD5)
-
1 cohort
21 HS and AD patients
75 mg (fed state)
(~equivalent exposure to 100mg
fasted MAD cohort dose level)
Open-label
28x daily doses
KYMERA ©2022 KYMERA THERAPEUTICS, INC.
●
●
●
●
Summary of Key Findings in MAD
IRAK4 degradation of 80-90% in PBMC using Flow Cytometry;
reduction to near lower limit of quantification with Mass Spectrometry
Associated with up to 85% inhibition of multiple disease-relevant
cytokines and chemokines in ex vivo TLR stimulation assay at 100 mg
dose
Dose-dependent IRAK4 degradation in skin of >50%
Generally well tolerated at doses up to 200 mg with no SAEs
Non-adverse, self-limiting QTcF prolongation in 10-20 msec range was
neither dose- nor exposure-dependent
Primary
Secondary/
Exploratory
●
Safety & tolerability
Pharmacokinetic measures
(half-life, bioavailability)
IRAK4 knockdown in PBMC and skin
Change in systemic inflammatory biomarkers and
proinflammatory gene transcripts in skin
Ex vivo response of whole blood to TLR agonists
Clinical endpoints: EASI (AD), Total AN Count (HS),
symptom scores and global assessments
PAGE 27View entire presentation