Bionomics IPO Presentation Deck
BNC210 is Potentially Well-Positioned in Post-Traumatic Stress Disorder
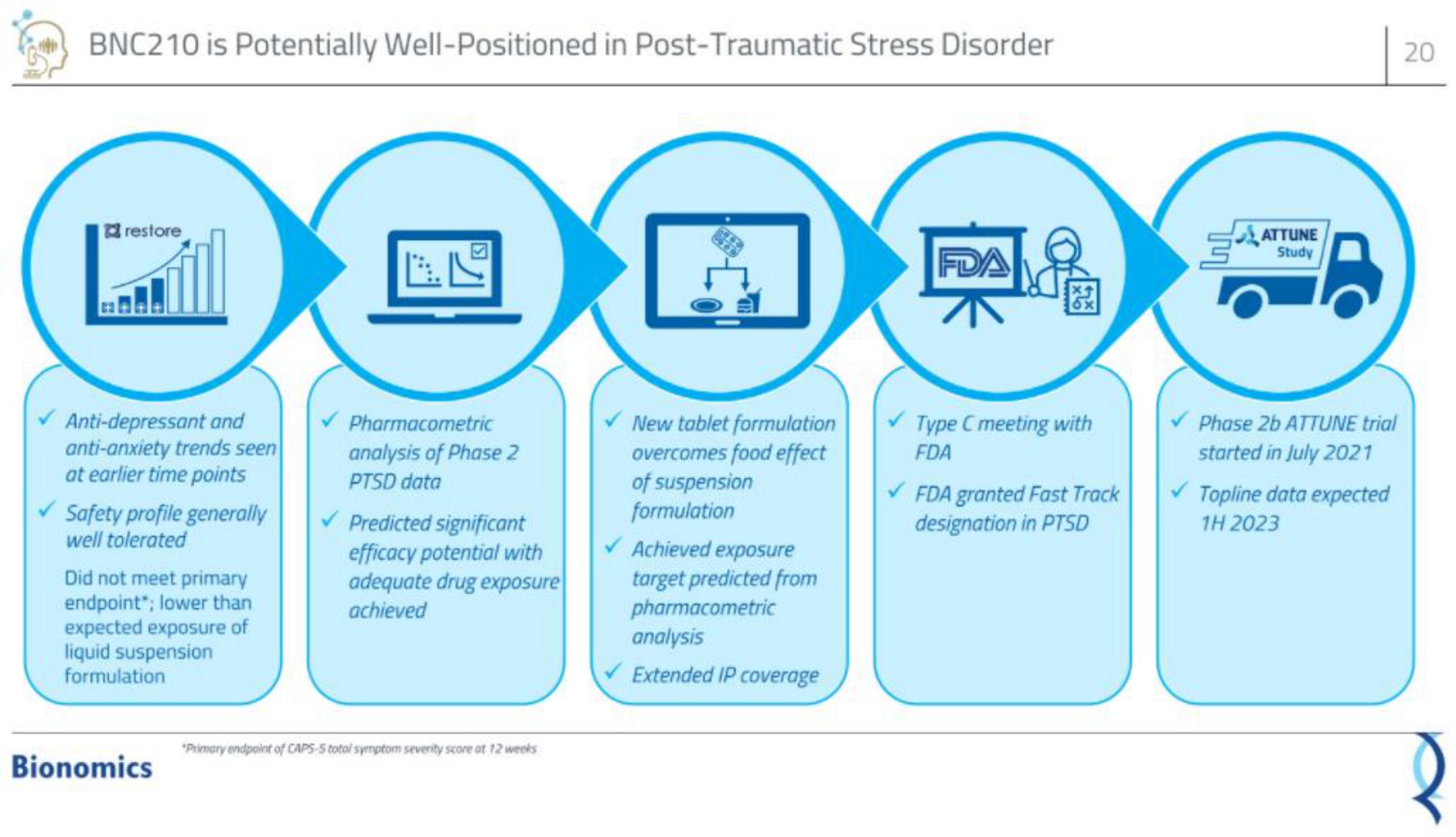
restore
Anti-depressant and
anti-anxiety trends seen
at earlier time points
✓Safety profile generall
well tolerated
Did not meet primary
endpoint"; lower than
expected exposure of
liquid suspension
formulation
Bionomics
NHA
✓New tablet formulation
overcomes food effect
of suspension
formulation
✓ Achieved exposure
target predicted from
pharmacometric
analysis
✓ Extended IP coverage
Pharmacometric
analysis of Phase 2
PTSD data
✓Predicted significant
efficacy potential with
adequate drug exposure
achieved
*Primary endpoint of CAPS-5 total symptom severity score at 12 weeks
FDA
Type C meeting with
FDA
✓ FDA granted Fast Track
designation in PTSD
ATTUNE
Study
✓ Phase 2b ATTUNE trial
started in July 2021
✓ Topline data expected
1H 2023
20View entire presentation