Dare Bioscience Investor Presentation Deck
ORGANON
BAYER
NIH
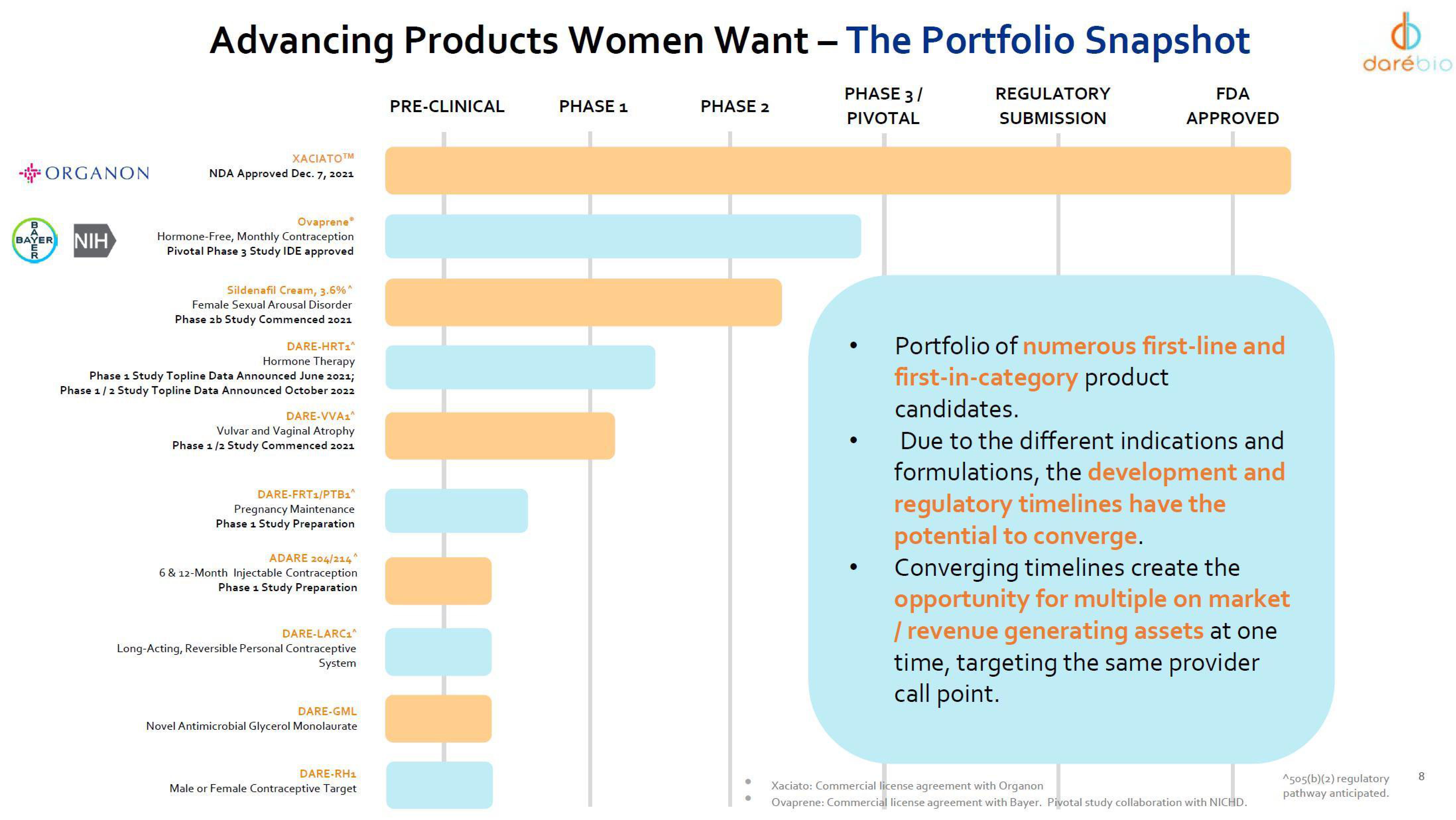
Advancing Products Women Want - The Portfolio Snapshot
PHASE 3/
PIVOTAL
ХАСIАТОТМ
NDA Approved Dec. 7, 2021
Ovaprene®
Hormone-Free, Monthly Contraception
Pivotal Phase 3 Study IDE approved
Sildenafil Cream, 3.6%^
Female Sexual Arousal Disorder
Phase 2b Study Commenced 2021
DARE-HRT₁^
Hormone Therapy
Phase 1 Study Topline Data Announced June 2021;
Phase 1/2 Study Topline Data Announced October 2022
DARE-VVA1^
Vulvar and Vaginal Atrophy
Phase 1/2 Study Commenced 2021
DARE-FRT1/PTB₁^
Pregnancy Maintenance
Phase 1 Study Preparation
ADARE 204/214^
6 & 12-Month Injectable Contraception
Phase 1 Study Preparation
DARE-LARC₁^
Long-Acting, Reversible Personal Contraceptive
System
DARE-GML
Novel Antimicrobial Glycerol Monolaurate
DARE-RH₁
Male or Female Contraceptive Target
PRE-CLINICAL
PHASE 1
PHASE 2
REGULATORY
SUBMISSION
FDA
APPROVED
Portfolio of numerous first-line and
first-in-category product
candidates.
Due to the different indications and
formulations, the development and
regulatory timelines have the
potential to converge.
Converging timelines create the
opportunity for multiple on market
/ revenue generating assets at one
time, targeting the same provider
call point.
Xaciato: Commercial license agreement with Organon
Ovaprene: Commercial license agreement with Bayer. Pivotal study collaboration with NICHD.
darébio
^505(b)(2) regulatory
pathway anticipated.View entire presentation