Kymera Investor Presentation Deck
●
●
●
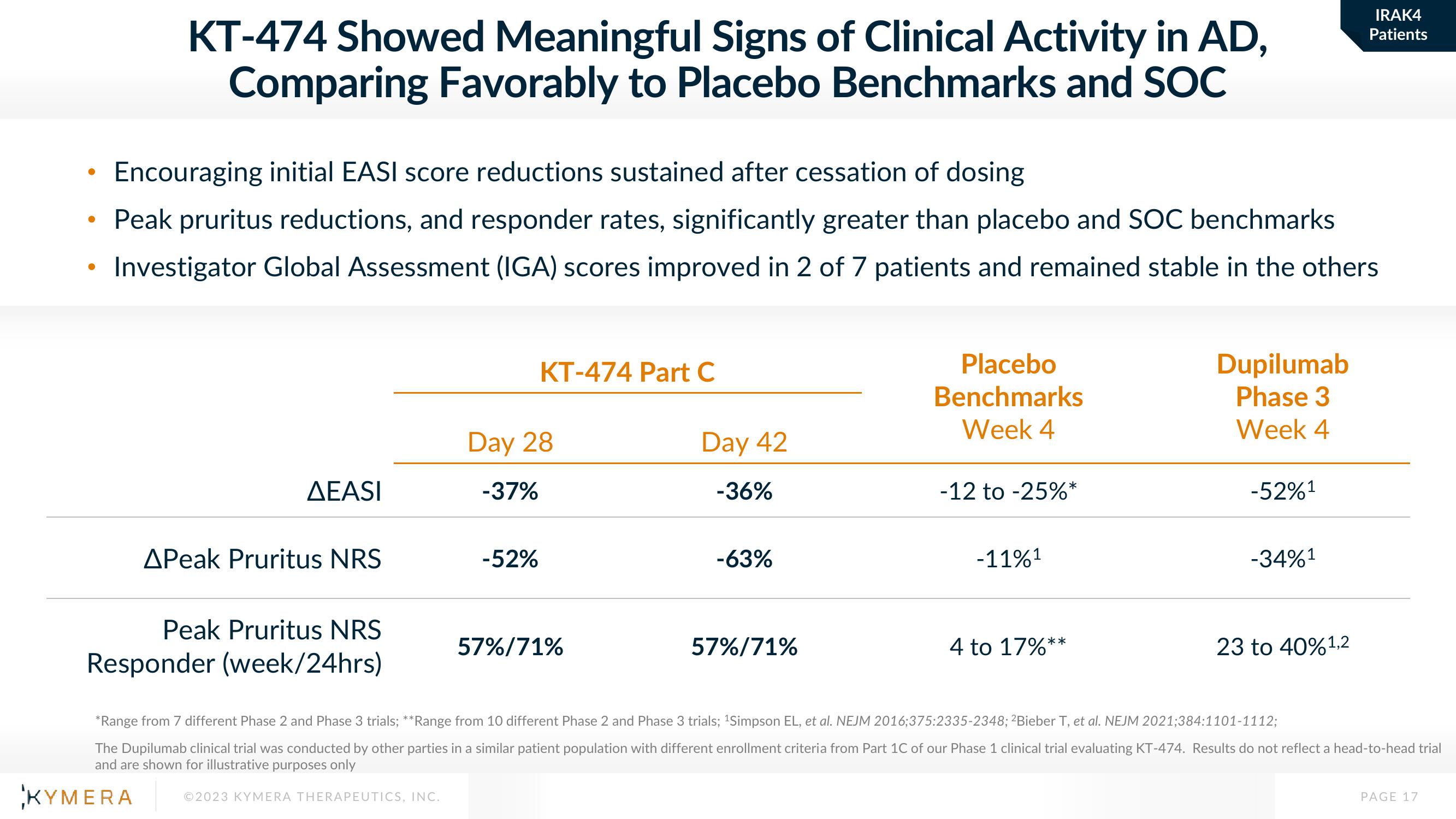
KT-474 Showed Meaningful Signs of Clinical Activity in AD,
Comparing Favorably to Placebo Benchmarks and SOC
Encouraging initial EASI score reductions sustained after cessation of dosing
Peak pruritus reductions, and responder rates, significantly greater than placebo and SOC benchmarks
Investigator Global Assessment (IGA) scores improved in 2 of 7 patients and remained stable in the others
AEASI
APeak Pruritus NRS
Peak Pruritus NRS
Responder (week/24hrs)
KT-474 Part C
Day 28
-37%
-52%
57%/71%
Day 42
-36%
-63%
57%/71%
Placebo
Benchmarks
Week 4
-12 to -25%*
-11%1
4 to 17%**
Dupilumab
Phase 3
Week 4
-52%¹
-34%¹
IRAK4
Patients
23 to 40%¹,2
*Range from 7 different Phase 2 and Phase 3 trials; **Range from 10 different Phase 2 and Phase 3 trials; ¹Simpson EL, et al. NEJM 2016;375:2335-2348; 2Bieber T, et al. NEJM 2021;384:1101-1112;
The Dupilumab clinical trial was conducted by other parties in a similar patient population with different enrollment criteria from Part 1C of our Phase 1 clinical trial evaluating KT-474. Results do not reflect a head-to-head trial
and are shown for illustrative purposes only
KYMERA Ⓒ2023 KYMERA THERAPEUTICS, INC.
PAGE 17View entire presentation