Context Therapeutics Investor Presentation Deck
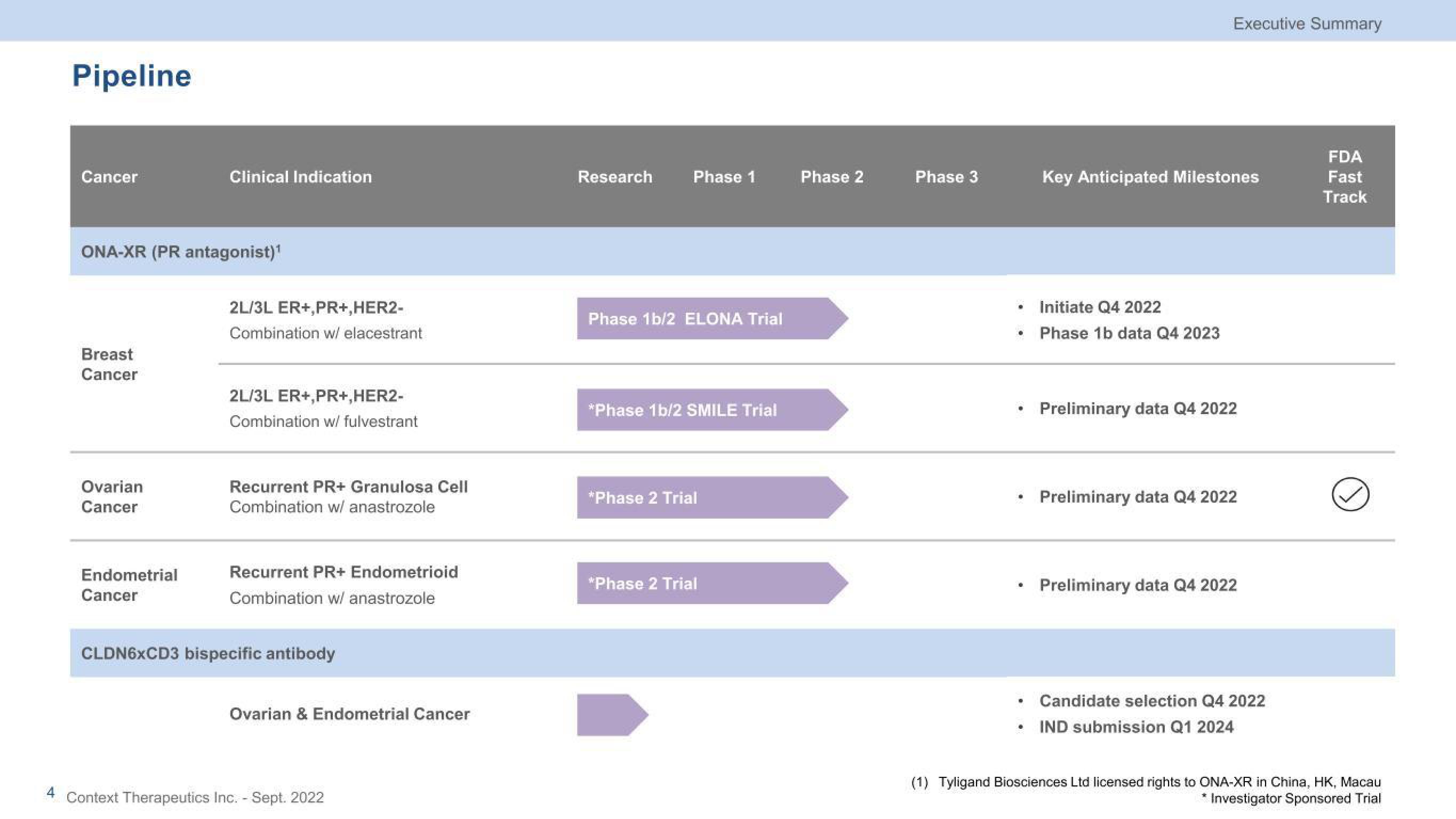
Pipeline
Cancer
ONA-XR (PR antagonist)¹
Breast
Cancer
Ovarian
Cancer
Clinical Indication
Endometrial
Cancer
2L/3L ER+,PR+, HER2-
Combination w/ elacestrant
2L/3L ER+,PR+,HER2-
Combination w/ fulvestrant
Recurrent PR+ Granulosa Cell
Combination w/ anastrozole
Recurrent PR+ Endometrioid
Combination w/ anastrozole
CLDN6xCD3 bispecific antibody
Ovarian & Endometrial Cancer
Context Therapeutics Inc. - Sept. 2022
Research
Phase 1
Phase 1b/2 ELONA Trial
*Phase 1b/2 SMILE Trial
*Phase 2 Trial
*Phase 2 Trial
Phase 2
Phase 3
Initiate Q4 2022
• Phase 1b data Q4 2023
.
.
.
Executive Summary
Key Anticipated Milestones
Preliminary data Q4 2022
Preliminary data Q4 2022
Preliminary data Q4 2022
Candidate selection Q4 2022
IND submission Q1 2024
FDA
Fast
Track
(1) Tyligand Biosciences Ltd licensed rights to ONA-XR in China, HK, Macau
* Investigator Sponsored TrialView entire presentation