Acquisition of APIRx
For personal use only
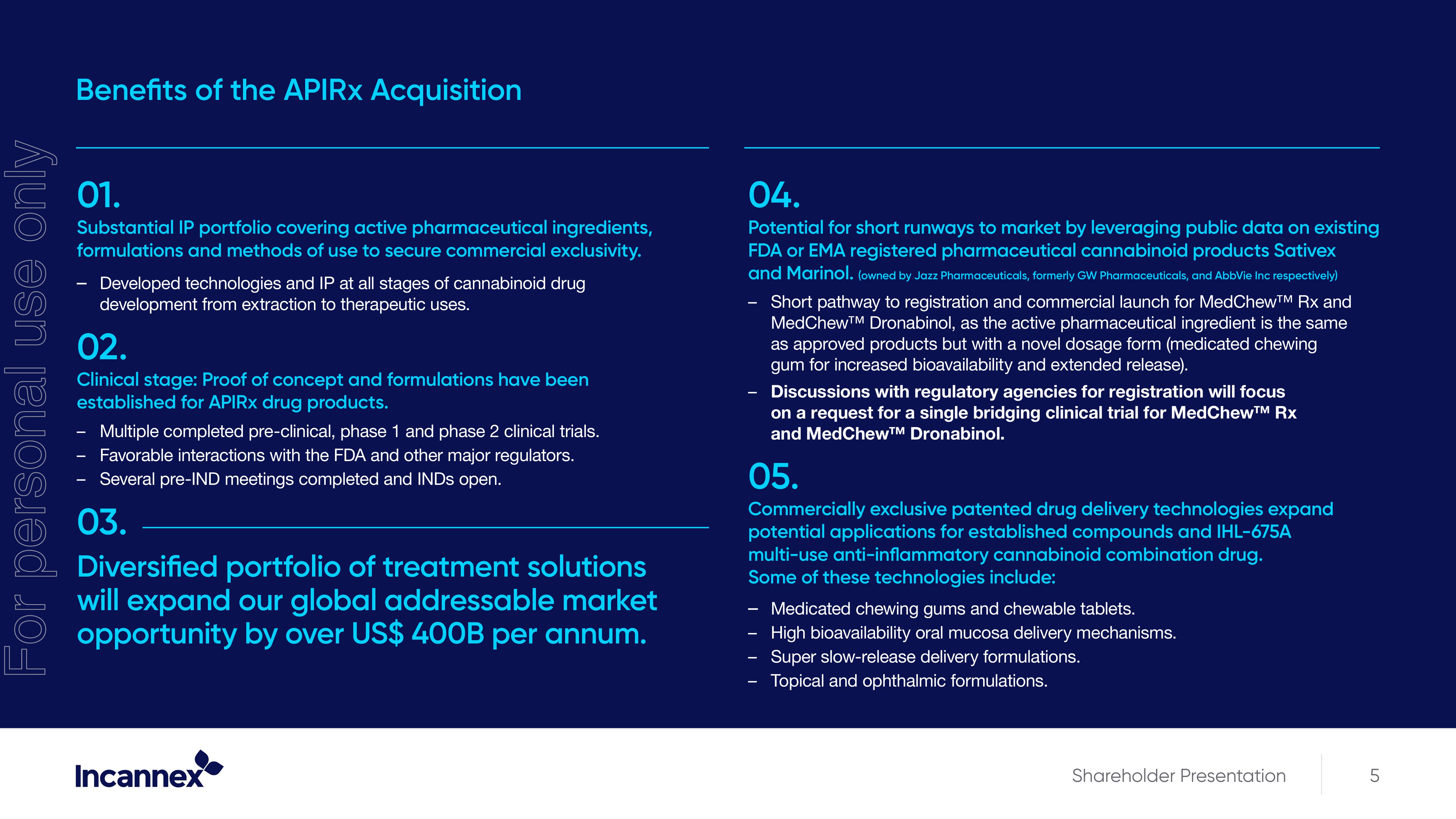
Benefits of the APIRx Acquisition
01.
Substantial IP portfolio covering active pharmaceutical ingredients,
formulations and methods of use to secure commercial exclusivity.
Developed technologies and IP at all stages of cannabinoid drug
development from extraction to therapeutic uses.
02.
Clinical stage: Proof of concept and formulations have been
established for APIRX drug products.
-
Multiple completed pre-clinical, phase 1 and phase 2 clinical trials.
Favorable interactions with the FDA and other major regulators.
Several pre-IND meetings completed and INDS open.
03.
Diversified portfolio of treatment solutions
will expand our global addressable market
opportunity by over US$ 400B per annum.
Incannex
04.
Potential for short runways to market by leveraging public data on existing
FDA or EMA registered pharmaceutical cannabinoid products Sativex
and Marinol. (owned by Jazz Pharmaceuticals, formerly GW Pharmaceuticals, and AbbVie Inc respectively)
Short pathway to registration and commercial launch for MedChew™ Rx and
MedChew™ Dronabinol, as the active pharmaceutical ingredient is the same
as approved products but with a novel dosage form (medicated chewing
gum for increased bioavailability and extended release).
Discussions with regulatory agencies for registration will focus
on a request for a single bridging clinical trial for MedChew™ Rx
and MedChew™ Dronabinol.
05.
Commercially exclusive patented drug delivery technologies expand
potential applications for established compounds and IHL-675A
multi-use anti-inflammatory cannabinoid combination drug.
Some of these technologies include:
Medicated chewing gums and chewable tablets.
High bioavailability oral mucosa delivery mechanisms.
Super slow-release delivery formulations.
Topical and ophthalmic formulations.
Shareholder Presentation
LO
5View entire presentation