AstraZeneca Investor Day Presentation Deck
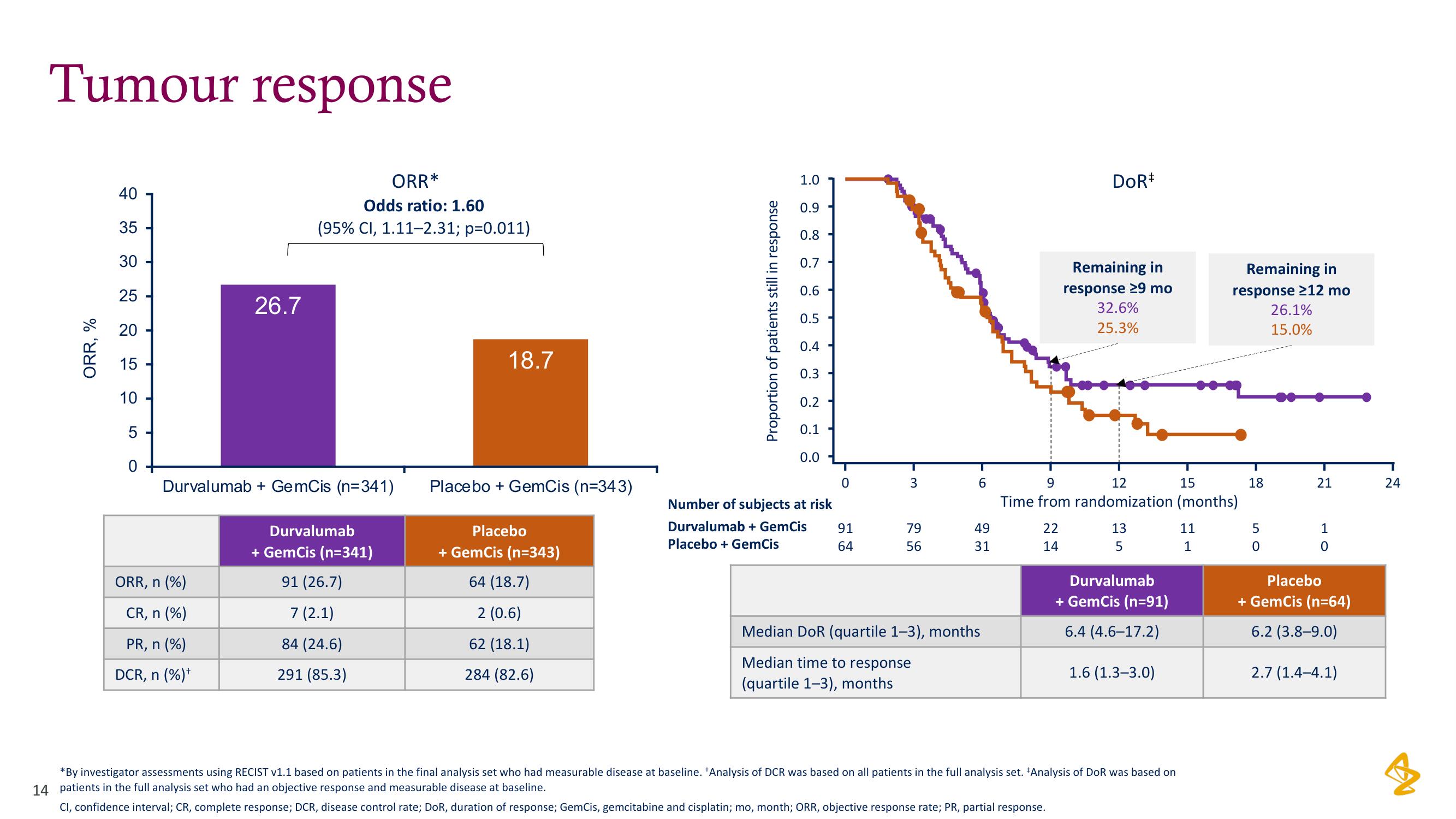
Tumour response
ORR, %
40
35
30
25
20
15
10
5
0
26.7
ORR, n (%)
CR, n (%)
PR, n (%)
DCR, n (%)*
ORR*
Odds ratio: 1.60
(95% CI, 1.11-2.31; p=0.011)
Durvalumab + GemCis (n=341)
Durvalumab
+ GemCis (n=341)
91 (26.7)
7 (2.1)
84 (24.6)
291 (85.3)
18.7
Placebo + GemCis (n=343)
Placebo
+ GemCis (n=343)
64 (18.7)
2 (0.6)
62 (18.1)
284 (82.6)
Proportion of patients still in response
1.0
0.9
0.8-
0.7 -
0.6
0.5-
0.4-
0.3
0.2
0.1
0.0
0
Number of subjects at risk
Durvalumab + GemCis 91
Placebo + GemCis
64
3
79
56
6
49
31
Median DoR (quartile 1–3), months
Median time to response
(quartile 1-3), months
DOR
22
14
Remaining in
response 29 mo
32.6%
25.3%
9
12
15
Time from randomization (months)
13
5
Durvalumab
+ GemCis (n=91)
6.4 (4.6-17.2)
1.6 (1.3-3.0)
*By investigator assessments using RECIST v1.1 based on patients in the final analysis set who had measurable disease at baseline. *Analysis of DCR was based on all patients in the full analysis set. *Analysis of DoR was based on
14 patients in the full analysis set who had an objective response and measurable disease at baseline.
CI, confidence interval; CR, complete response; DCR, disease control rate; DoR, duration of response; GemCis, gemcitabine and cisplatin; mo, month; ORR, objective response rate; PR, partial response.
Remaining in
response ≥12 mo
26.1%
15.0%
11
1
18
5
0
21
1
0
Placebo
+ GemCis (n=64)
6.2 (3.8-9.0)
2.7 (1.4-4.1)
24
3View entire presentation