Certara Investor Presentation Deck
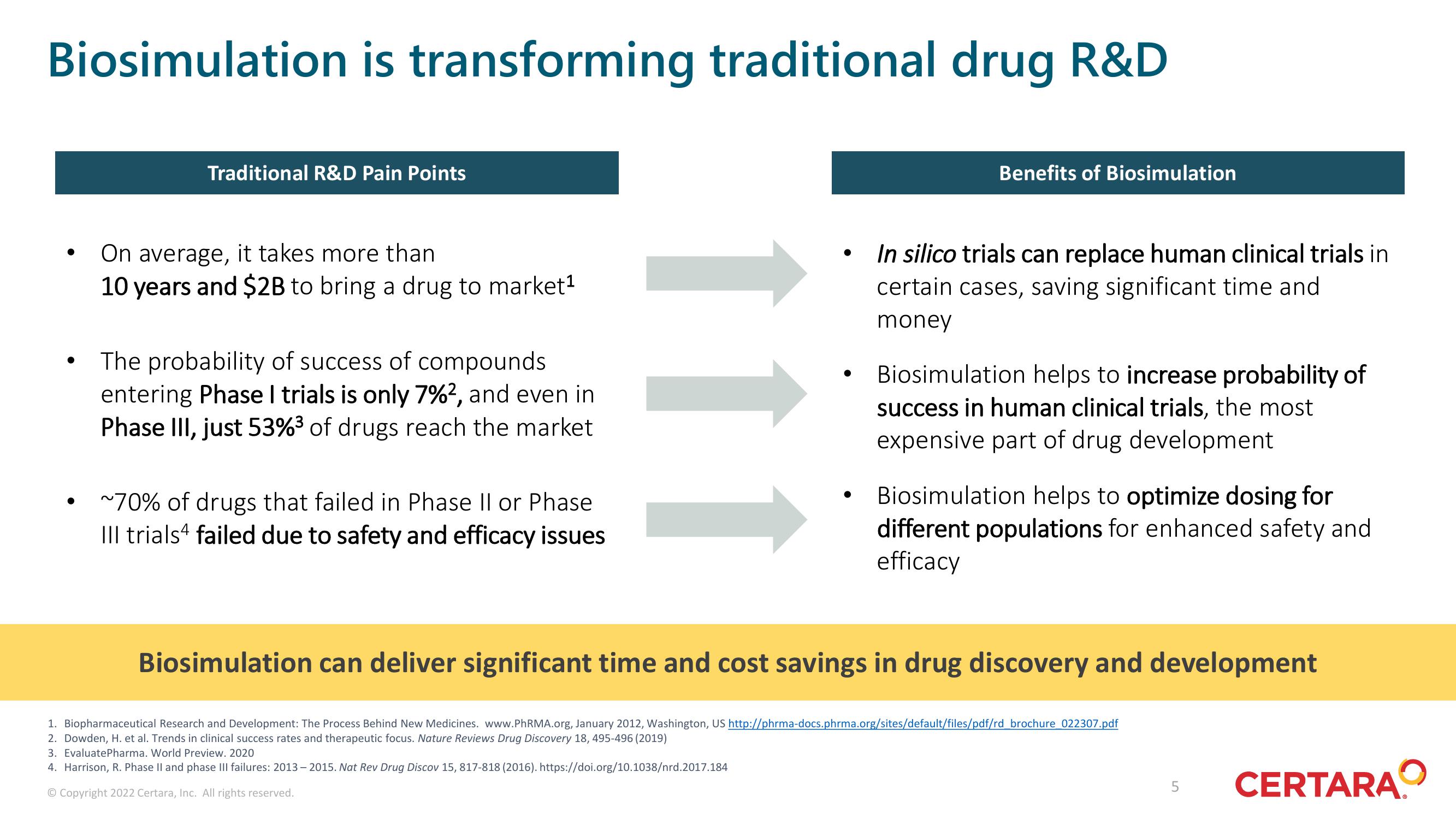
Biosimulation is transforming traditional drug R&D
●
Traditional R&D Pain Points
On average, it takes more than
10 years and $2B to bring a drug to market¹
The probability of success of compounds
entering Phase I trials is only 7%², and even in
Phase III, just 53%³ of drugs reach the market
~70% of drugs that failed in Phase II or Phase
III trials4 failed due to safety and efficacy issues
111
●
Benefits of Biosimulation
In silico trials can replace human clinical trials in
certain cases, saving significant time and
money
Biosimulation helps to increase probability of
success in human clinical trials, the most
expensive part of drug development
Biosimulation helps to optimize dosing for
different populations for enhanced safety and
efficacy
Biosimulation can deliver significant time and cost savings in drug discovery and development
1. Biopharmaceutical Research and Development: The Process Behind New Medicines. www.PhRMA.org, January 2012, Washington, US http://phrma-docs.phrma.org/sites/default/files/pdf/rd brochure 022307.pdf
2. Dowden, H. et al. Trends in clinical success rates and therapeutic focus. Nature Reviews Drug Discovery 18, 495-496 (2019)
3. EvaluatePharma. World Preview. 2020
4. Harrison, R. Phase II and phase III failures: 2013-2015. Nat Rev Drug Discov 15, 817-818 (2016). https://doi.org/10.1038/nrd.2017.184
© Copyright 2022 Certara, Inc. All rights reserved.
5
CERTARAView entire presentation