Centessa IPO Presentation Deck
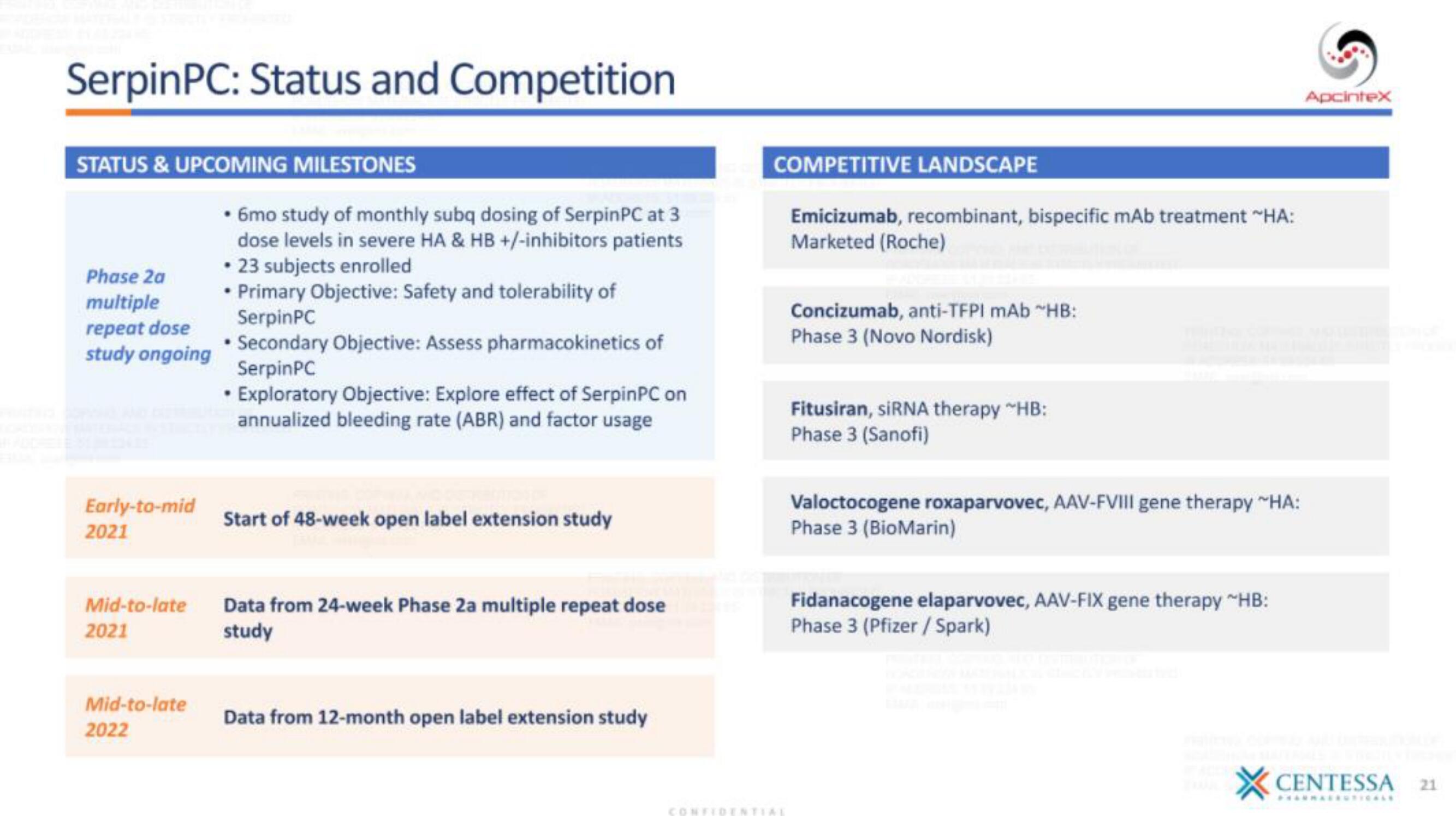
SerpinPC: Status and Competition
STATUS & UPCOMING MILESTONES
Phase 2a
multiple
repeat dose
study ongoing
Early-to-mid
2021
Mid-to-late
2021
Mid-to-late
2022
• 6mo study of monthly subq dosing of SerpinPC at 3
dose levels in severe HA & HB +/-inhibitors patients
• 23 subjects enrolled
• Primary Objective: Safety and tolerability of
SerpinPC
• Secondary Objective: Assess pharmacokinetics of
SerpinPC
• Exploratory Objective: Explore effect of SerpinPC on
annualized bleeding rate (ABR) and factor usage
Start of 48-week open label extension study
Data from 24-week Phase 2a multiple repeat dose
study
Data from 12-month open label extension study
COMPETITIVE LANDSCAPE
Emicizumab, recombinant, bispecific mAb treatment ~HA:
Marketed (Roche)
Concizumab, anti-TFPI mAb ~HB:
Phase 3 (Novo Nordisk)
Fitusiran, siRNA therapy "HB:
Phase 3 (Sanofi)
Valoctocogene roxaparvovec, AAV-FVIII gene therapy ~HA:
Phase 3 (BioMarin)
Fidanacogene elaparvovec, AAV-FIX gene therapy ~HB:
Phase 3 (Pfizer / Spark)
Apcintex
CENTESSA 21View entire presentation