BioAtla Investor Presentation Deck
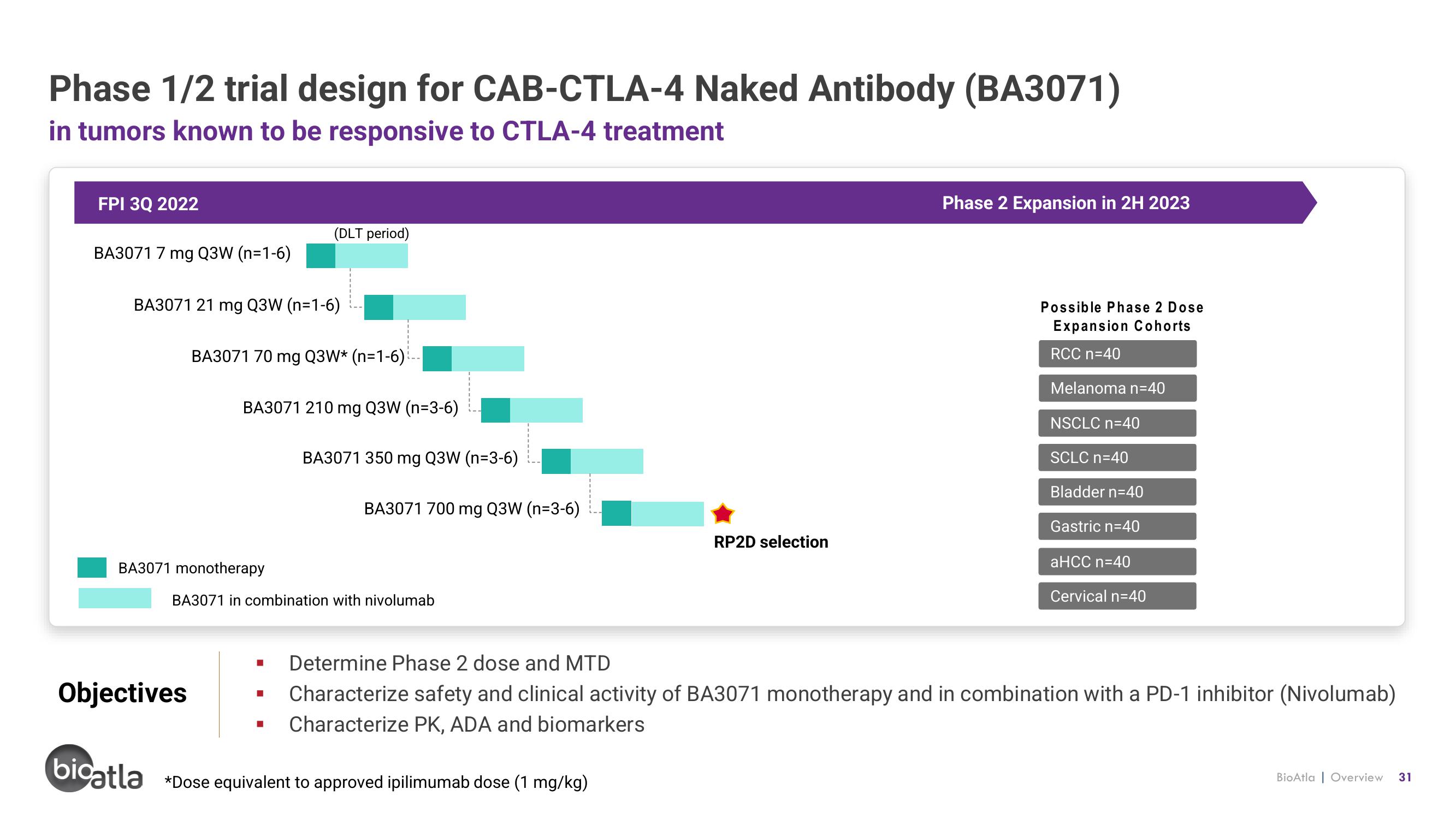
Phase 1/2 trial design for CAB-CTLA-4 Naked Antibody (BA3071)
in tumors known to be responsive to CTLA-4 treatment
FPI 3Q 2022
BA3071 7 mg Q3W (n=1-6)
BA3071 21 mg Q3W (n=1-6)
BA3071 70 mg Q3W* (n=1-6)
BA3071 monotherapy
Objectives
(DLT period)
BA3071 210 mg Q3W (n=3-6)
BA3071 in combination with nivolumab
I
BA3071 350 mg Q3W (n-3-6)
I
BA3071 700 mg Q3W (n-3-6)
RP2D selection
bicatla *Dose equivalent to approved ipilimumab dose (1 mg/kg)
Phase 2 Expansion in 2H 2023
Possible Phase 2 Dose
Expansion Cohorts
RCC n=40
Melanoma n=40
NSCLC n=40
SCLC n=40
Bladder n=40
■ Determine Phase 2 dose and MTD
Characterize safety and clinical activity of BA3071 monotherapy and in combination with a PD-1 inhibitor (Nivolumab)
Characterize PK, ADA and biomarkers
Gastric n=40
aHCC n=40
Cervical n=40
BioAtla| Overview
31View entire presentation