BioAtla Investor Presentation Deck
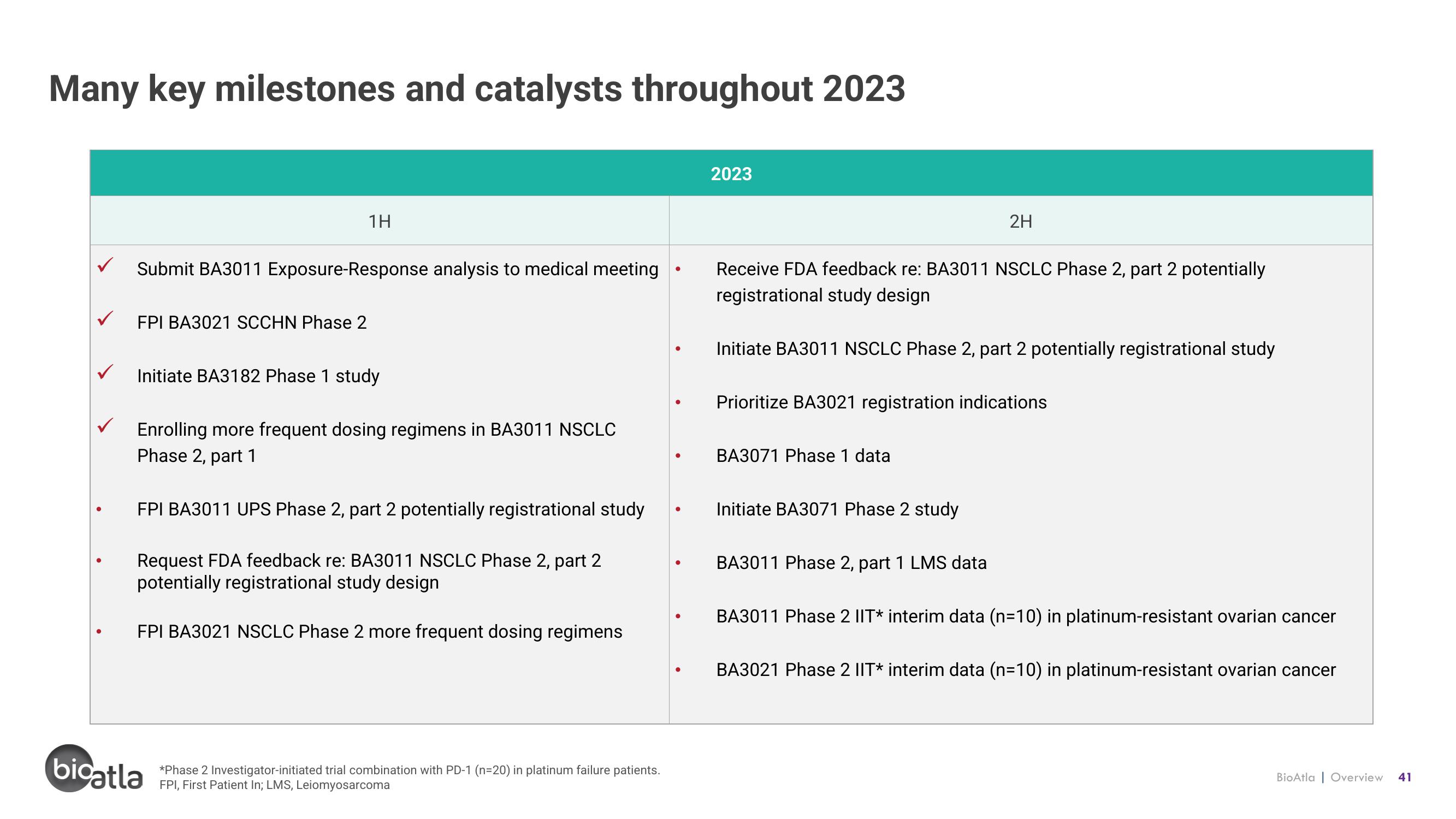
Many key milestones and catalysts throughout 2023
Submit BA3011 Exposure-Response analysis to medical meeting
FPI BA3021 SCCHN Phase 2
1H
Initiate BA3182 Phase 1 study
Enrolling more frequent dosing regimens in BA3011 NSCLC
Phase 2, part 1
FPI BA3011 UPS Phase 2, part 2 potentially registrational study
Request FDA feedback re: BA3011 NSCLC Phase 2, part 2
potentially registrational study design
FPI BA3021 NSCLC Phase 2 more frequent dosing regimens
bicatla
*Phase 2 Investigator-initiated trial combination with PD-1 (n=20) in platinum failure patients.
FPI, First Patient In; LMS, Leiomyosarcoma
2023
Receive FDA feedback re: BA3011 NSCLC Phase 2, part 2 potentially
registrational study design
Initiate BA3011 NSCLC Phase 2, part 2 potentially registrational study
2H
Prioritize BA3021 registration indications
BA3071 Phase 1 data
Initiate BA3071 Phase 2 study
BA3011 Phase 2, part 1 LMS data
BA3011 Phase 2 IIT* interim data (n=10) in platinum-resistant ovarian cancer
BA3021 Phase 2 IIT* interim data (n=10) in platinum-resistant ovarian cancer
BioAtla| Overview 41View entire presentation