Dare Bioscience Investor Presentation Deck
1.
2.
3.
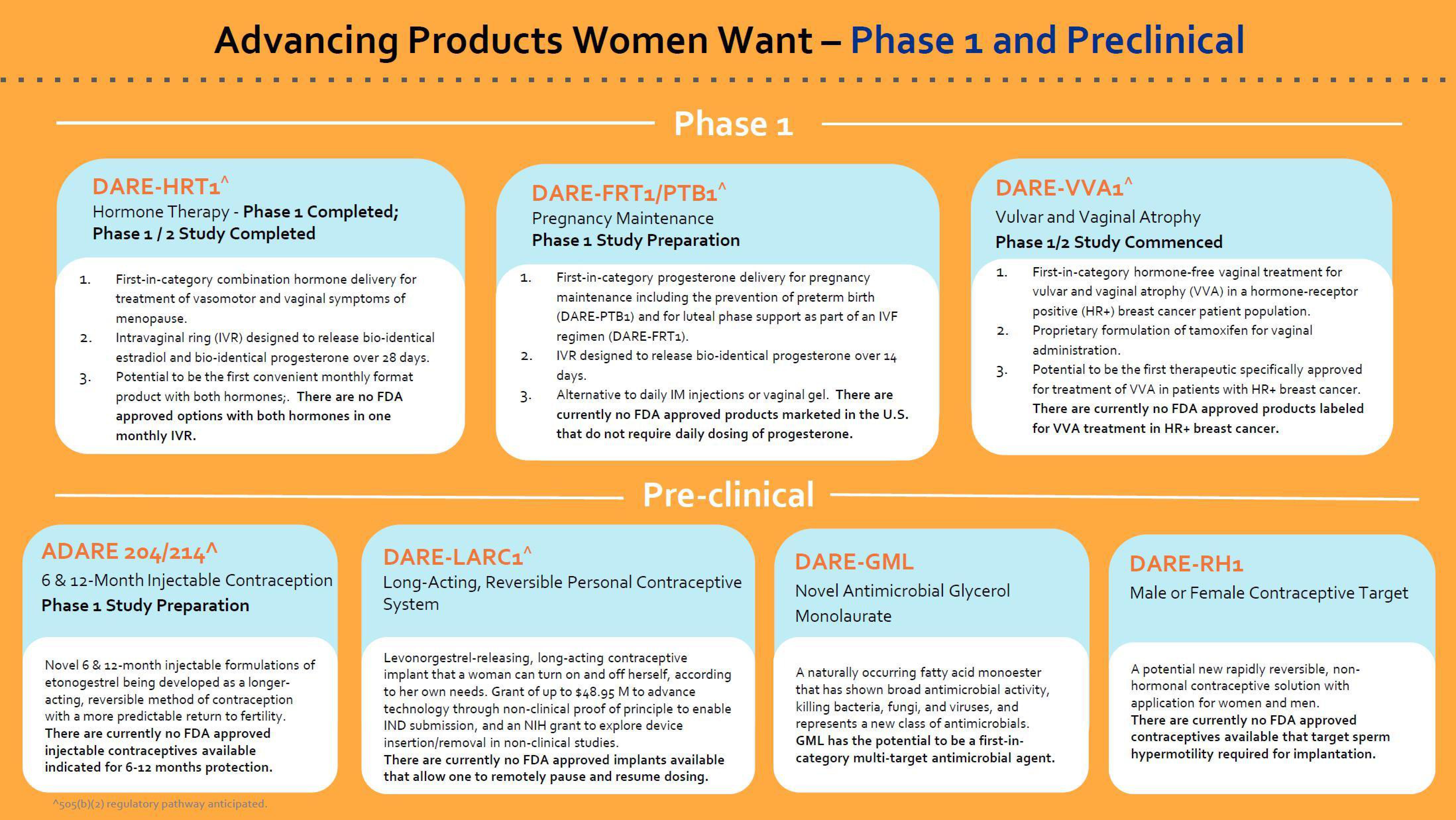
Advancing Products Women Want - Phase 1 and Preclinical
DARE-HRT1^
Hormone Therapy - Phase 1 Completed;
Phase 1/2 Study Completed
First-in-category combination hormone delivery for
treatment of vasomotor and vaginal symptoms of
menopause.
Intravaginal ring (IVR) designed to release bio-identical
estradiol and bio-identical progesterone over 28 days.
Potential to be the first convenient monthly format
product with both hormones;. There are no FDA
approved options with both hormones in one
monthly IVR.
ADARE 204/214^
6 & 12-Month Injectable Contraception
Phase 1 Study Preparation
Novel 6 & 12-month injectable formulations of
etonogestrel being developed as a longer-
acting, reversible method of contraception
with a more predictable return to fertility.
There are currently no FDA approved
injectable contraceptives available
indicated for 6-12 months protection.
^505(b)(2) regulatory pathway anticipated.
1.
DARE-FRT1/PTB1^
Pregnancy Maintenance
Phase 1 Study Preparation
2.
Phase 1
3.
First-in-category progesterone delivery for pregnancy
maintenance including the prevention of preterm birth
(DARE-PTB1) and for luteal phase support as part of an IVF
regimen (DARE-FR 1).
IVR designed to release bio-identical progesterone over 14
days.
Alternative to daily IM injections or vaginal gel. There are
currently no FDA approved products marketed in the U.S.
that do not require daily dosing of progesterone.
Pre-clinical
DARE-LARC1^
Long-Acting, Reversible Personal Contraceptive
System
Levonorgestrel-releasing, long-acting contraceptive
implant that a woman can turn on and off herself, according
to her own needs. Grant of up to $48.95 M to advance
technology through non-clinical proof of principle to enable
IND submission, and an NIH grant to explore device
insertion/removal in non-clinical studies.
There are currently no FDA approved implants available
that allow one to remotely pause and resume dosing.
DARE-VVA1^
Vulvar and Vaginal Atrophy
Phase 1/2 Study Commenced
1.
2.
3.
DARE-GML
Novel Antimicrobial Glycerol
Monolaurate
First-in-category hormone-free vaginal treatment for
vulvar and vaginal atrophy (VVA) in a hormone-receptor
positive (HR+) breast cancer patient population.
Proprietary formulation of tamoxifen for vaginal
administration.
☐☐☐☐☐
Potential to be the first therapeutic specifically approved
for treatment of VVA in patients with HR+ breast cancer.
There are currently no FDA approved products labeled
for VVA treatment in HR+ breast cancer.
A naturally occurring fatty acid monoester
that has shown broad antimicrobial activity,
killing bacteria, fungi, and viruses, and
represents a new class of antimicrobials.
GML has the potential to be a first-in-
category multi-target antimicrobial agent.
DARE-RH1
Male or Female Contraceptive Target
A potential new rapidly reversible, non-
hormonal contraceptive solution with
application for women and men.
There are currently no FDA approved
contraceptives available that target sperm
hypermotility required for implantation.View entire presentation