BioNTech Results Presentation Deck
50% serum neutralizing titer
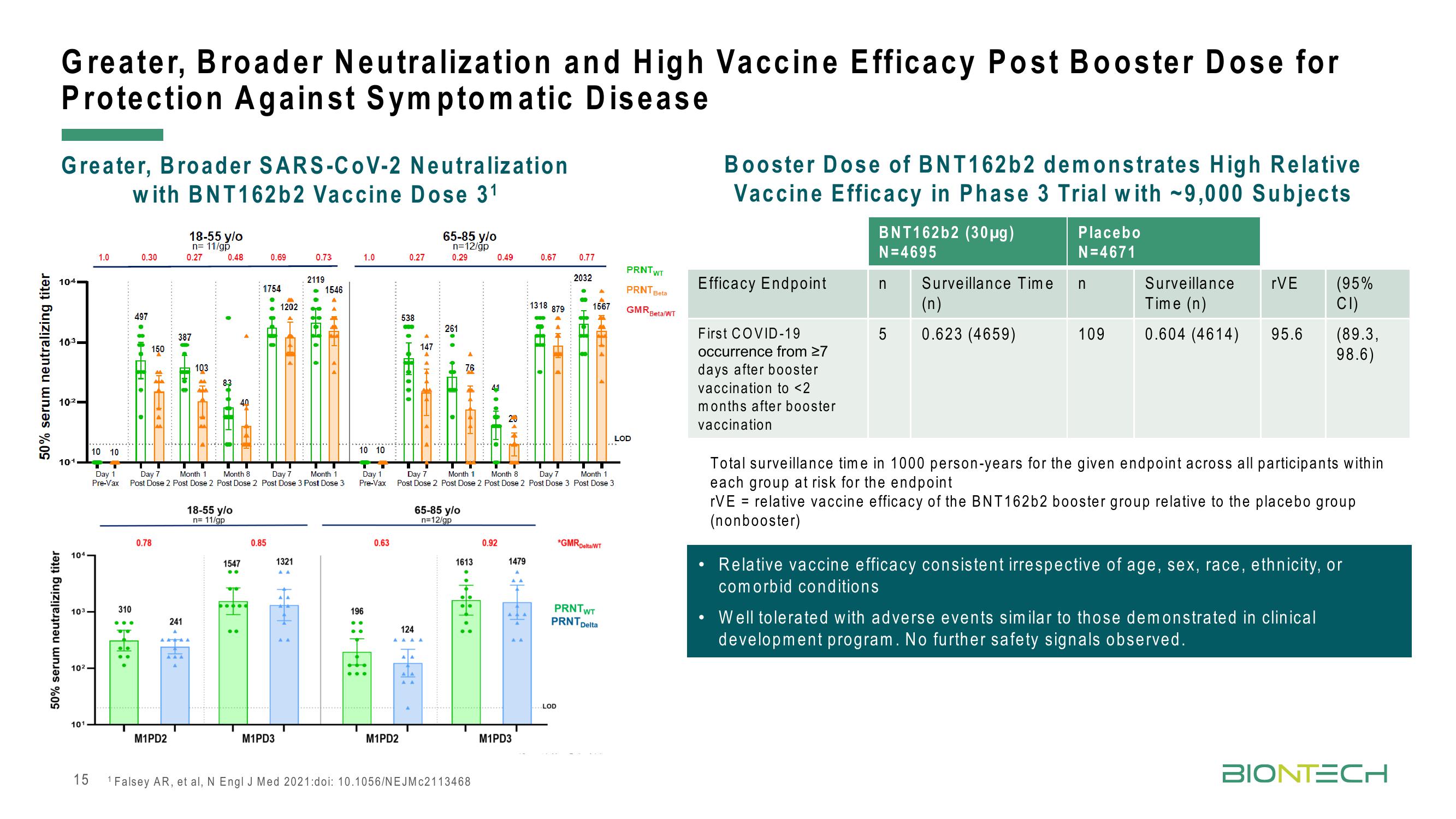
Greater, Broader Neutralization and High Vaccine Efficacy Post Booster Dose for
Protection Against Symptomatic Disease
Greater, Broader SARS-CoV-2 Neutralization
with BNT162b2 Vaccine Dose 31
104
103
10²
101
103-
10²
101
1.0
10 10
Day 1
Pre-Vax
310
0.30
T
497
.
150
${
0.78
M1PD2
18-55 y/o
n= 11/gp
0.27
387
241
103
0.48
1
Month 8
18-55 y/o
n= 11/gp
1547
..
0.69
1754
Day 7
Month 1
Day 7
Month 1
Post Dose 2 Post Dose 2 Post Dose 2 Post Dose 3 Post Dose 3
0.85
1202
M1PD3
0.73
1321
2119
•!•
1546
HE 44
1.0
10 10
Day 1
Pre-Vax
196
0.63
0.27
M1PD2
538
124
147
AA
A A
65-85 y/o
n=12/gp
0.29
261
HED
76
65-85 y/o
n=12/gp
1613
0.49
15
1 Falsey AR, et al, N Engl J Med 2021:doi: 10.1056/NEJMC2113468
2 H
Month 1
Day 7 Month 1 Month 8 Day 7
Post Dose 2 Post Dose 2 Post Dose 2 Post Dose 3 Post Dose 3
0.92
1479
0.67
M1PD3
1318 879
THE
0.77
2032
LOD
1567
*GMR Delta/WT
PRNTWT
PRNT Delta
PRNTWT
PRNT
GMR Beta/WT
LOD
Beta
Booster Dose of BNT162b2 demonstrates High Relative
Vaccine Efficacy in Phase 3 Trial with ~9,000 Subjects
Efficacy Endpoint
First COVID-19
occurrence from ≥7
days after booster
vaccination to <2
months after booster
vaccination
●
BNT162b2 (30µg)
N=4695
n
5
Surveillance Time
(n)
0.623 (4659)
Placebo
N=4671
n
109
Surveillance rVE
Time (n)
0.604 (4614)
95.6
(95%
CI)
(89.3,
98.6)
Total surveillance time in 1000 person-years for the given endpoint across all participants within
each group at risk for the endpoint
rVE = relative vaccine efficacy of the BNT162b2 booster group relative to the placebo group
(nonbooster)
Well tolerated with adverse events similar to those demonstrated in clinical
development program. No further safety signals observed.
Relative vaccine efficacy consistent irrespective of age, sex, race, ethnicity, or
comorbid conditions
BIONTECHView entire presentation