BioNTech Results Presentation Deck
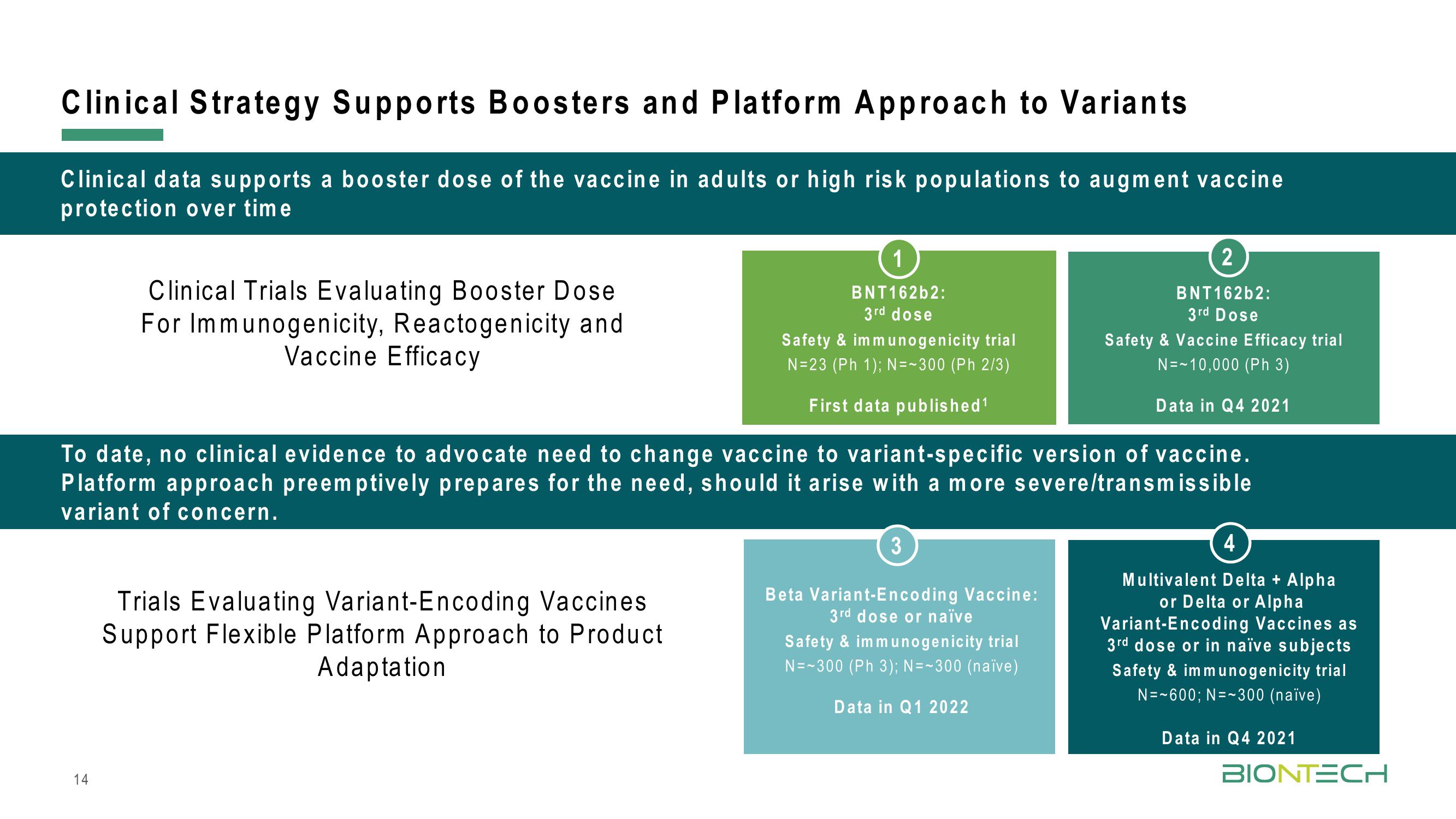
Clinical Strategy Supports Boosters and Platform Approach to Variants
Clinical data supports a booster dose of the vaccine in adults or high risk populations to augment vaccine
protection over time
Clinical Trials Evaluating Booster Dose
For Immunogenicity, Reactogenicity and
Vaccine Efficacy
14
1
BNT162b2:
3rd dose
Trials Evaluating Variant-Encoding Vaccines
Support Flexible Platform Approach to Product
Adaptation
Safety & immunogenicity trial
N=23 (Ph 1); N=~300 (Ph 2/3)
First data published ¹
To date, no clinical evidence to advocate need to change vaccine to variant-specific version of vaccine.
Platform approach preemptively prepares for the need, should it arise with a more severe/transmissible
variant of concern.
3
Beta Variant-Encoding Vaccine:
3rd dose or naïve
Safety & immunogenicity trial
N=~300 (Ph 3); N=~300 (naïve)
2
BNT162b2:
3rd Dose
Safety & Vaccine Efficacy trial
N=~10,000 (Ph 3)
Data in Q4 2021
Data in Q1 2022
4
Multivalent Delta + Alpha
or Delta or Alpha
Variant-Encoding Vaccines as
3rd dose or in naïve subjects
Safety & immunogenicity trial
N=~600; N= 300 (naïve)
Data in Q4 2021
BIONTECHView entire presentation