Aravive Investor Presentation Deck
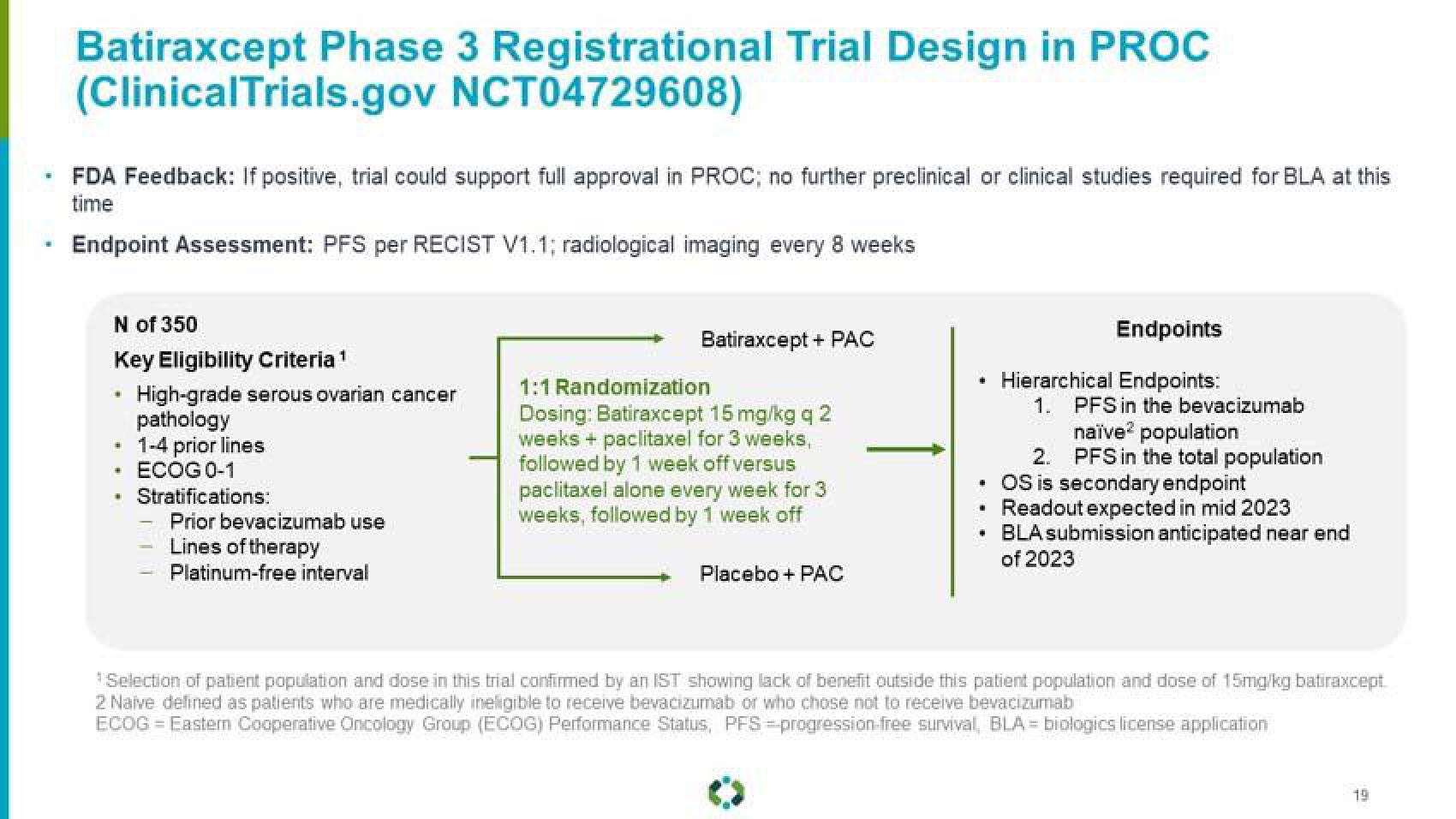
Batiraxcept Phase 3 Registrational Trial Design in PROC
(Clinical Trials.gov NCT04729608)
FDA Feedback: If positive, trial could support full approval in PROC; no further preclinical or clinical studies required for BLA at this
time
Endpoint Assessment: PFS per RECIST V1.1; radiological imaging every 8 weeks
N of 350
Key Eligibility Criteria
High-grade serous ovarian cancer
pathology
1-4 prior lines
ECOG 0-1
i
i
.
Stratifications:
Prior bevacizumab use
Lines of therapy
Platinum-free interval
Batiraxcept + PAC
1:1 Randomization
Dosing: Batiraxcept 15 mg/kg q 2
weeks + paclitaxel for 3 weeks,
followed by 1 week off versus
paclitaxel alone every week for 3
weeks, followed by 1 week off
Placebo + PAC
Endpoints
+ Hierarchical Endpoints:
1.
PFS in the bevacizumab
naïve2 population
PFS in the total population
2.
. OS is secondary endpoint
Readout expected in mid 2023
. BLA submission anticipated near end
of 2023
* Selection of patient population and dose in this trial confirmed by an IST showing lack of benefit outside this patient population and dose of 15mg/kg batiraxcept
2 Naive defined as patients who are medically ineligible to receive bevacizumab or who chose not to receive bevacizumab
ECOG = Eastern Cooperative Oncology Group (ECOG) Performance Status, PFS-progression-free survival, BLA= biologics license application
19View entire presentation