Certara Investor Presentation Deck
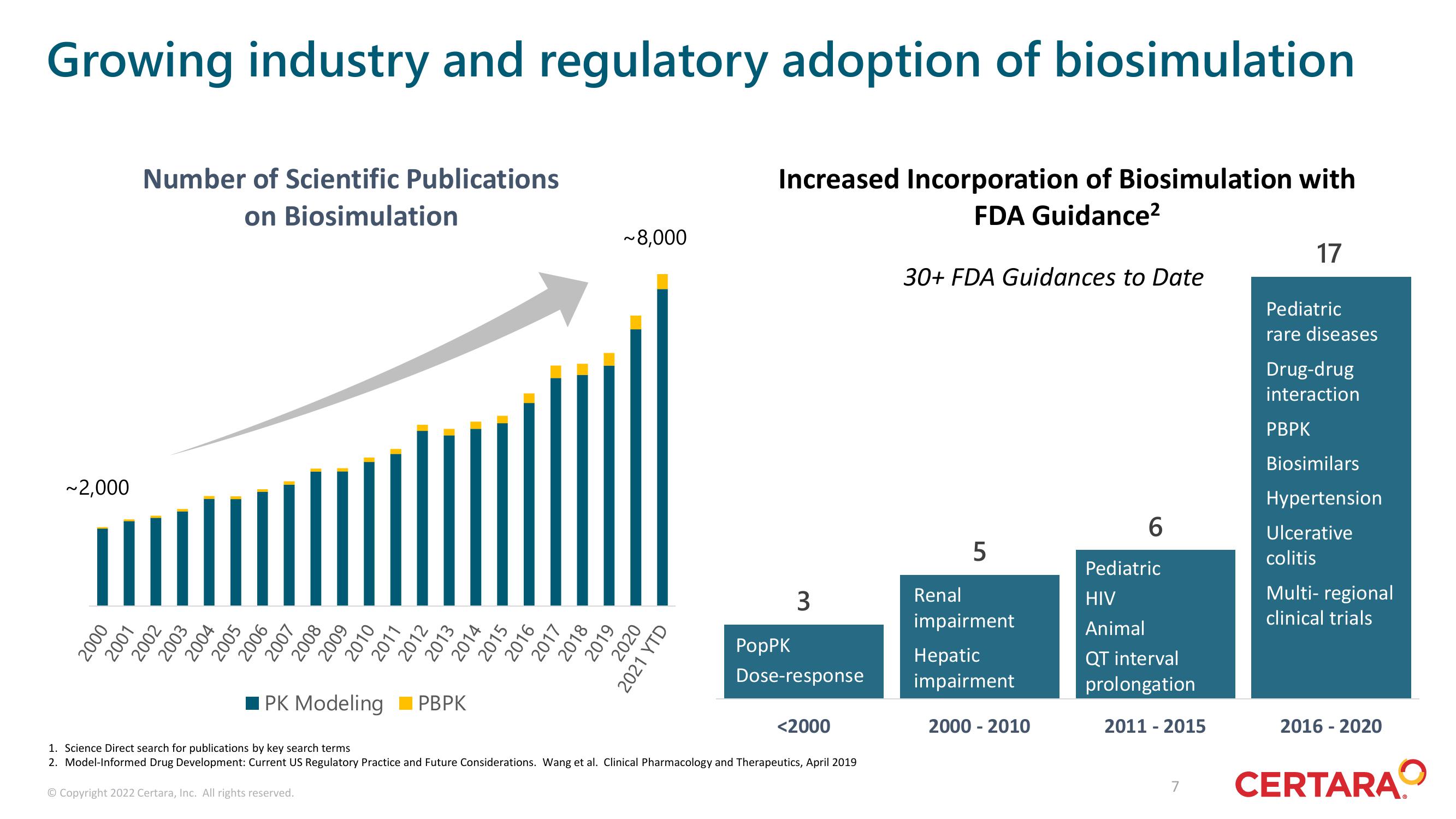
Growing industry and regulatory adoption of biosimulation
Increased Incorporation of Biosimulation with
FDA Guidance²
~2,000
2000
2001
Number of Scientific Publications
on
Biosimulation
2002
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
PK Modeling PBPK
2014
2015
2016
2017
2018
2019
~8,000
2020
2021 YTD
PopPK
3
Dose-response
<2000
1. Science Direct search for publications by key search terms
2. Model-Informed Drug Development: Current US Regulatory Practice and Future Considerations. Wang et al. Clinical Pharmacology and Therapeutics, April 2019
© Copyright 2022 Certara, Inc. All rights reserved.
30+ FDA Guidances to Date
5
Renal
impairment
Hepatic
impairment
2000 - 2010
6
Pediatric
HIV
Animal
QT interval
prolongation
2011-2015
7
17
Pediatric
rare diseases
Drug-drug
interaction
PBPK
Biosimilars
Hypertension
Ulcerative
colitis
Multi-regional
clinical trials
2016-2020
CERTARAView entire presentation