Calliditas Therapeutics IPO Presentation Deck
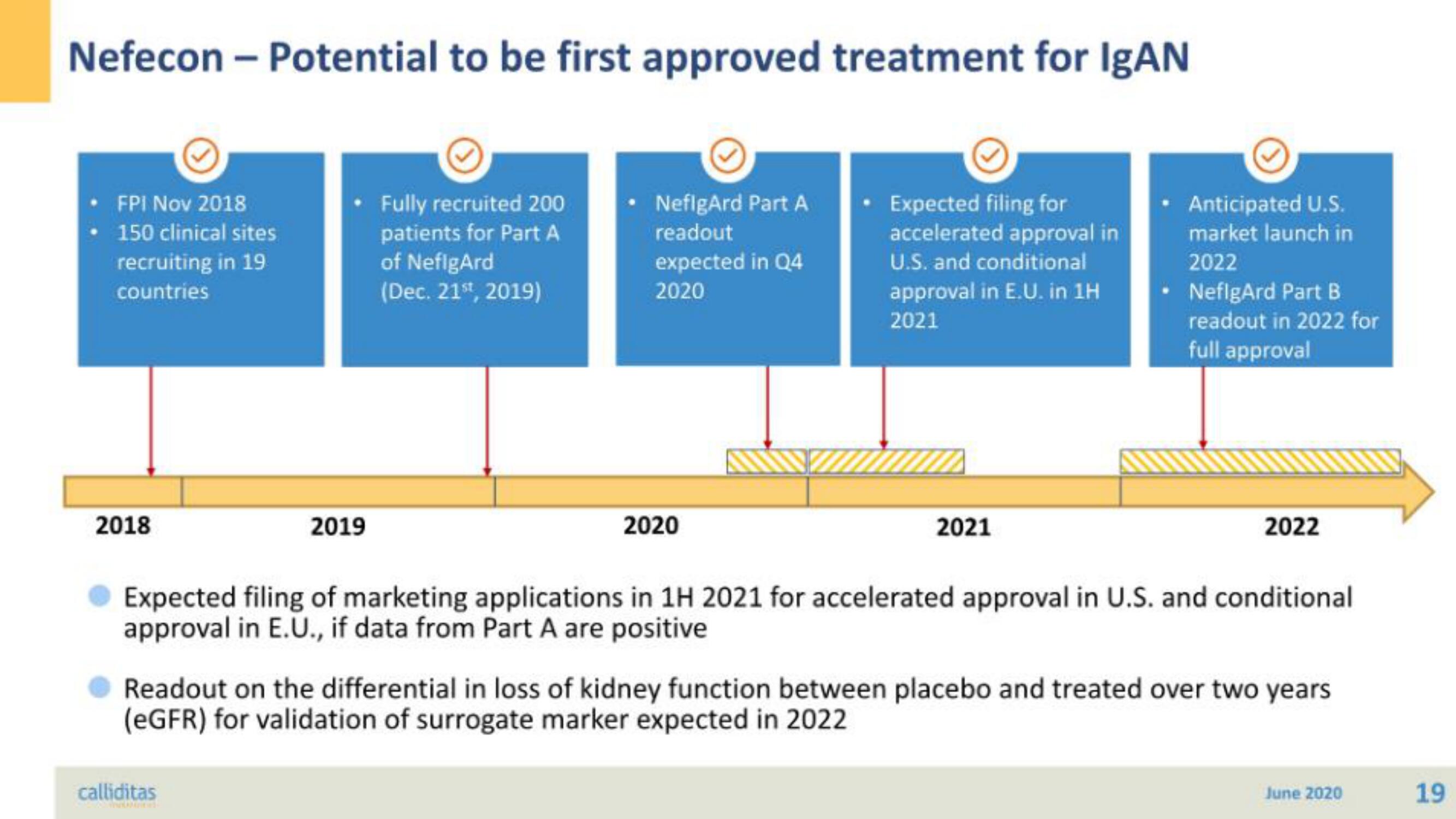
Nefecon - Potential to be first approved treatment for IgAN
FPI Nov 2018
150 clinical sites
recruiting in 19
countries
2018
2019
Fully recruited 200
patients for Part A
of NefigArd
(Dec. 21st, 2019)
calliditas
NeflgArd Part A
readout
expected in Q4
2020
2020
Expected filing for
accelerated approval in
U.S. and conditional
approval in E.U. in 1H
2021
2021
Anticipated U.S.
market launch in
2022
NeflgArd Part B
readout in 2022 for
full approval
2022
Expected filing of marketing applications in 1H 2021 for accelerated approval in U.S. and conditional
approval in E.U., if data from Part A are positive
Readout on the differential in loss of kidney function between placebo and treated over two years
(eGFR) for validation of surrogate marker expected in 2022
June 2020
19View entire presentation