Akouos IPO Presentation Deck
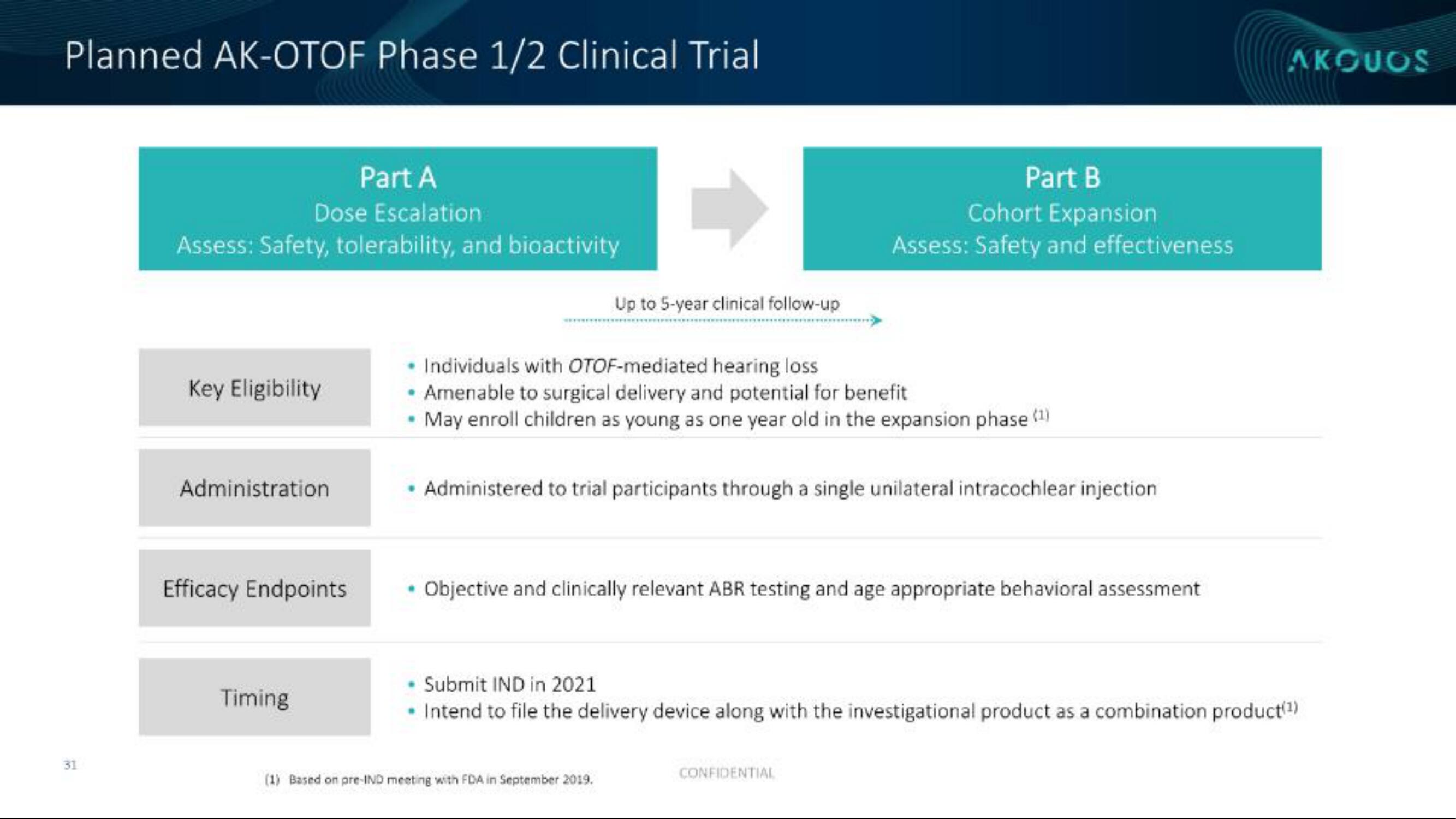
Planned AK-OTOF Phase 1/2 Clinical Trial
31
Part A
Dose Escalation
Assess: Safety, tolerability, and bioactivity
Key Eligibility
Administration
Efficacy Endpoints
Timing
Up to 5-year clinical follow-up
Individuals with OTOF-mediated hearing loss
• Amenable to surgical delivery and potential for benefit
• May enroll children as young as one year old in the expansion phase (¹)
Part B
Cohort Expansion
Assess: Safety and effectiveness
Administered to trial participants through a single unilateral intracochlear injection
• Objective and clinically relevant ABR testing and age appropriate behavioral assessment
(1) Based on pre-IND meeting with FDA in September 2019.
Submit IND in 2021
• Intend to file the delivery device along with the investigational product as a combination product(¹)
CONFIDENTIAL
AKQUOSView entire presentation