Nuvectis Pharma Investor Presentation Deck
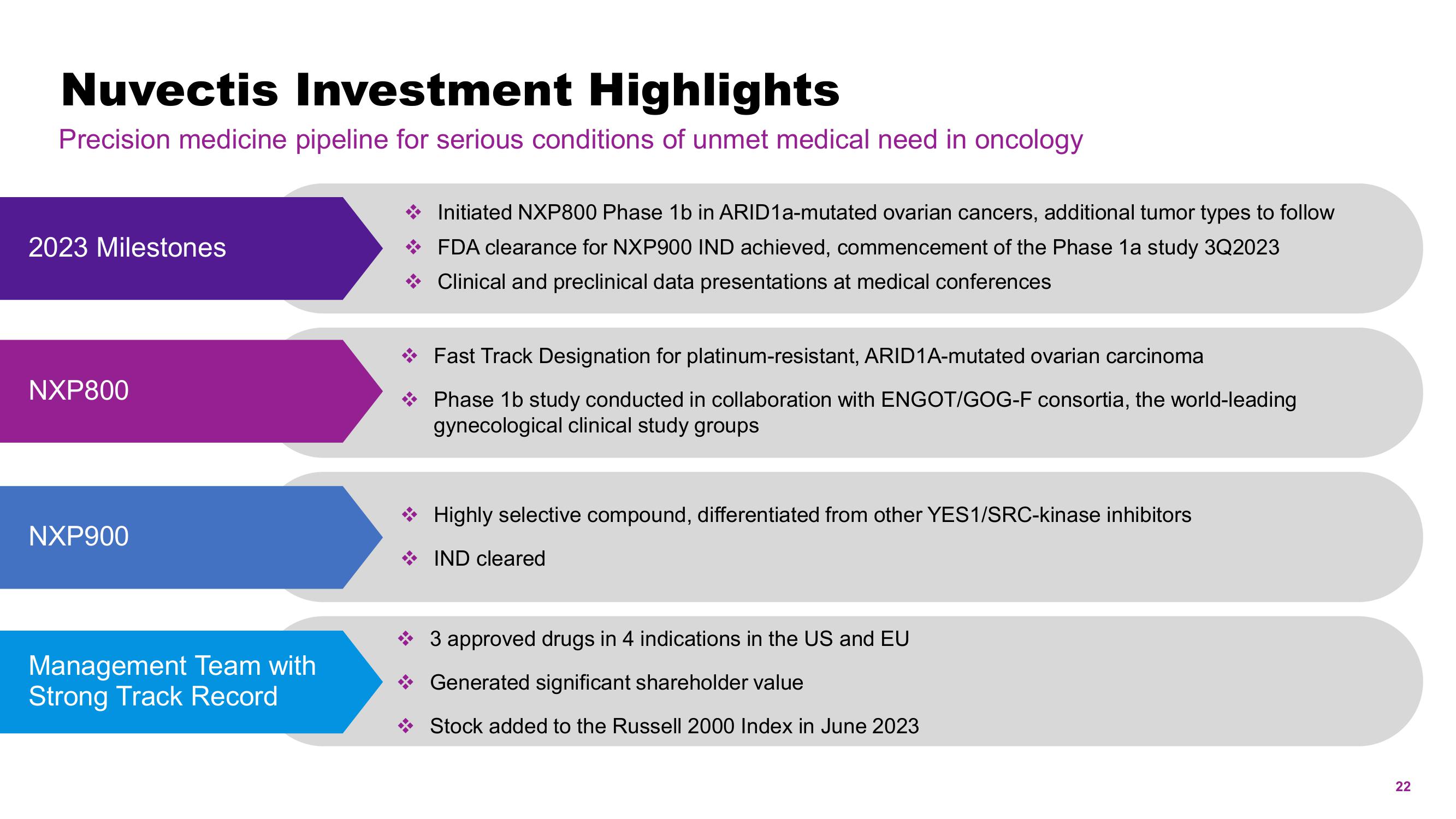
Nuvectis Investment Highlights
Precision medicine pipeline for serious conditions of unmet medical need in oncology
2023 Milestones
NXP800
NXP900
Management Team with
Strong Track Record
→ Fast Track Designation for platinum-resistant, ARID1A-mutated ovarian carcinoma
Phase 1b study conducted in collaboration with ENGOT/GOG-F consortia, the world-leading
gynecological clinical study groups
O
Initiated NXP800 Phase 1b in ARID1a-mutated ovarian cancers, additional tumor types to follow
FDA clearance for NXP900 IND achieved, commencement of the Phase 1a study 3Q2023
Clinical and preclinical data presentations at medical conferences
T
Highly selective compound, differentiated from other YES1/SRC-kinase inhibitors
IND cleared
3 approved drugs in 4 indications in the US and EU
Generated significant shareholder value
Stock added to the Russell 2000 Index in June 2023
22View entire presentation