BioAtla IPO Presentation Deck
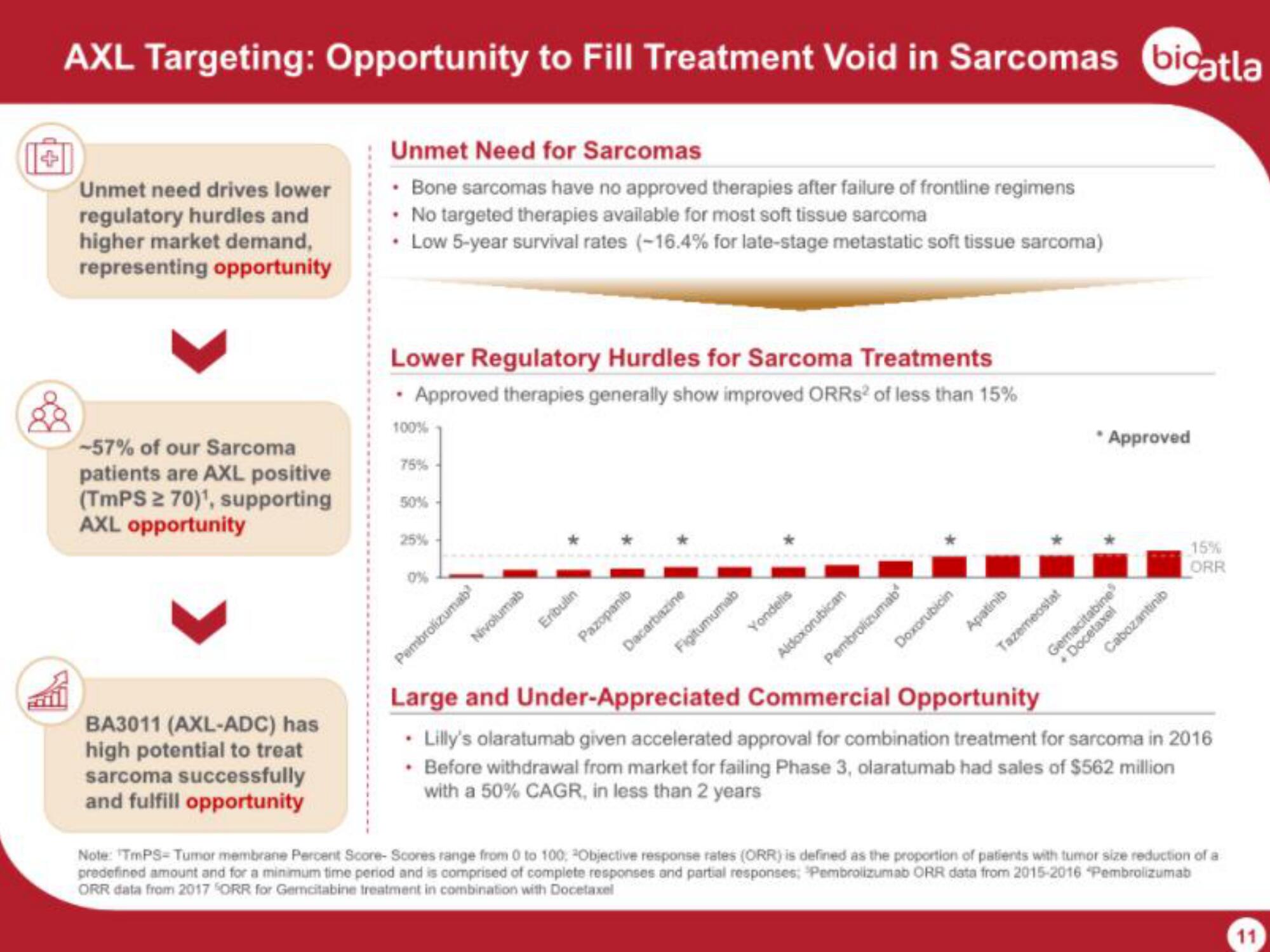
AXL Targeting: Opportunity to Fill Treatment Void in Sarcomas bicatla
Unmet need drives lower
regulatory hurdles and
higher market demand,
representing opportunity
-57% of our Sarcoma
patients are AXL positive
(TmPS ≥ 70)¹, supporting
AXL opportunity
BA3011 (AXL-ADC) has
high potential to treat
sarcoma successfully
and fulfill opportunity
Unmet Need for Sarcomas
• Bone sarcomas have no approved therapies after failure of frontline regimens
• No targeted therapies available for most soft tissue sarcoma
• Low 5-year survival rates (-16.4% for late-stage metastatic soft tissue sarcoma)
Lower Regulatory Hurdles for Sarcoma Treatments
• Approved therapies generally show improved ORRS² of less than 15%
100%
75%
50%-
25%
Pembrolizumab
Nivolumab
Eribulin
Pazopanib
Dacarbazine
Figitumumab
Aldoxorubican
Yondelis
Pembrolizumab
////
Doxorubicin
Apatinib
Tazemeostat
* Approved
Gemacitabine
*Docetaxel
Cabozantinib
15%
ORR
Large and Under-Appreciated Commercial Opportunity
• Lilly's olaratumab given accelerated approval for combination treatment for sarcoma in 2016
• Before withdrawal from market for failing Phase 3, olaratumab had sales of $562 million
with a 50% CAGR, in less than 2 years
Note: TmPS-Tumor membrane Percent Score- Scores range from 0 to 100, "Objective response rates (ORR) is defined as the proportion of patients with tumor size reduction of a
predefined amount and for a minimum time period and is comprised of complete responses and partial responses; Pembrolizumab ORR data from 2015-2016 Pembrolizumab
ORR data from 2017 SORR for Gemcitabine treatment in combination with Docetaxel
11View entire presentation