Kymera Investor Day Presentation Deck
●
●
●
●
●
●
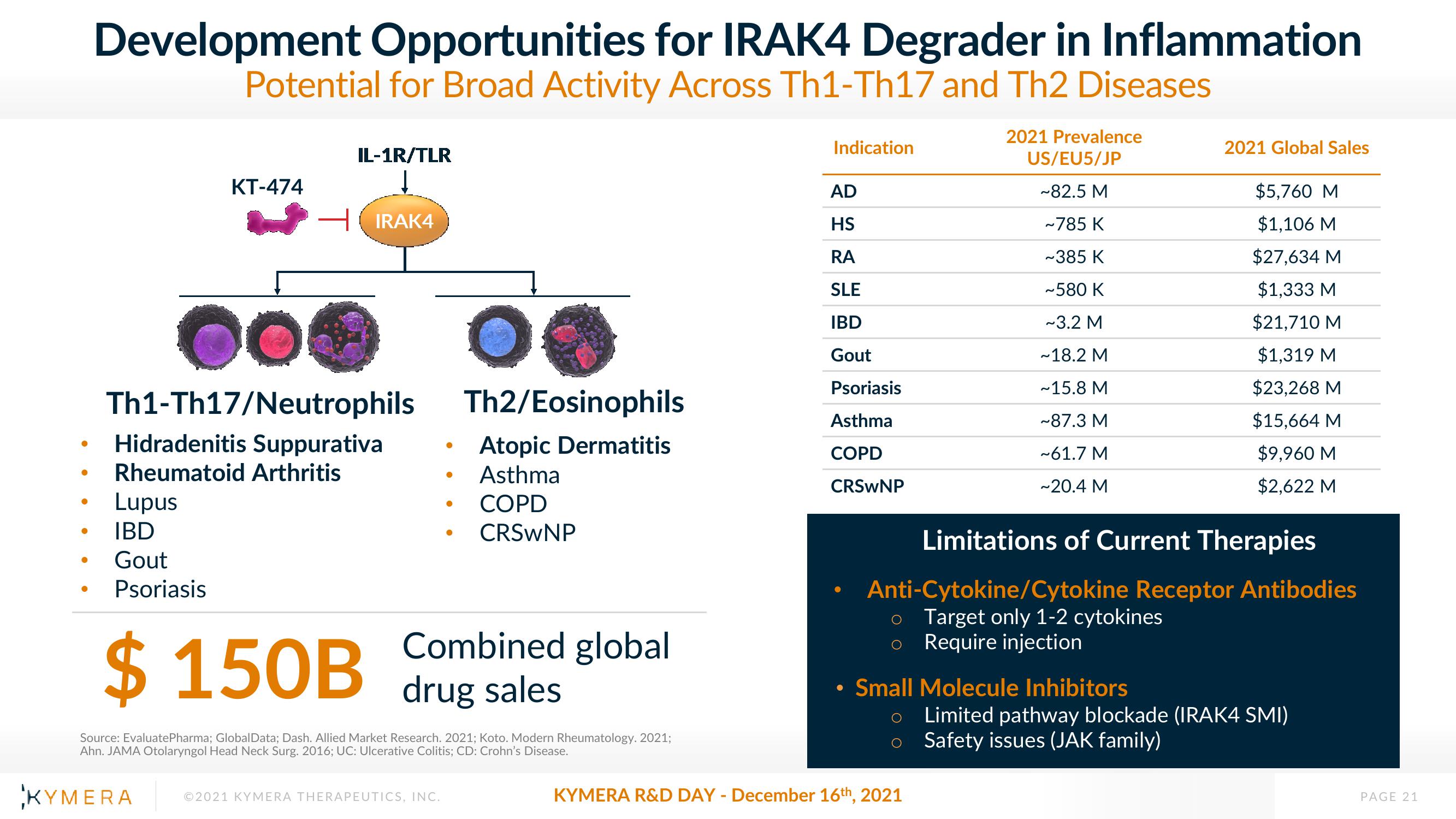
Development Opportunities for IRAK4 Degrader in Inflammation
Potential for Broad Activity Across Th1-Th17 and Th2 Diseases
KT-474
IL-1R/TLR
Lupus
IBD
Gout
Psoriasis
IRAK4
OC
Th1-Th17/Neutrophils
Hidradenitis Suppurativa
Rheumatoid Arthritis
●
●
●
●
Th2/Eosinophils
Atopic Dermatitis
Asthma
COPD
CRSWNP
$150B drug sales
Source: EvaluatePharma; GlobalData; Dash. Allied Market Research. 2021; Koto. Modern Rheumatology. 2021;
Ahn. JAMA Otolaryngol Head Neck Surg. 2016; UC: Ulcerative Colitis; CD: Crohn's Disease.
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
Combined global
Indication
AD
HS
RA
SLE
IBD
Gout
Psoriasis
Asthma
COPD
CRSWNP
●
2021 Prevalence
US/EU5/JP
~82.5 M
~785 K
~385 K
~580 K
~3.2 M
~18.2 M
~15.8 M
~87.3 M
~61.7 M
~20.4 M
Small Molecule Inhibitors
o
o
KYMERA R&D DAY - December 16th, 2021
2021 Global Sales
Limitations of Current Therapies
Anti-Cytokine/Cytokine Receptor Antibodies
o Target only 1-2 cytokines
o Require injection
$5,760 M
$1,106 M
$27,634 M
$1,333 M
$21,710 M
$1,319 M
$23,268 M
$15,664 M
$9,960 M
$2,622 M
Limited pathway blockade (IRAK4 SMI)
Safety issues (JAK family)
PAGE 21View entire presentation