Imara M&A
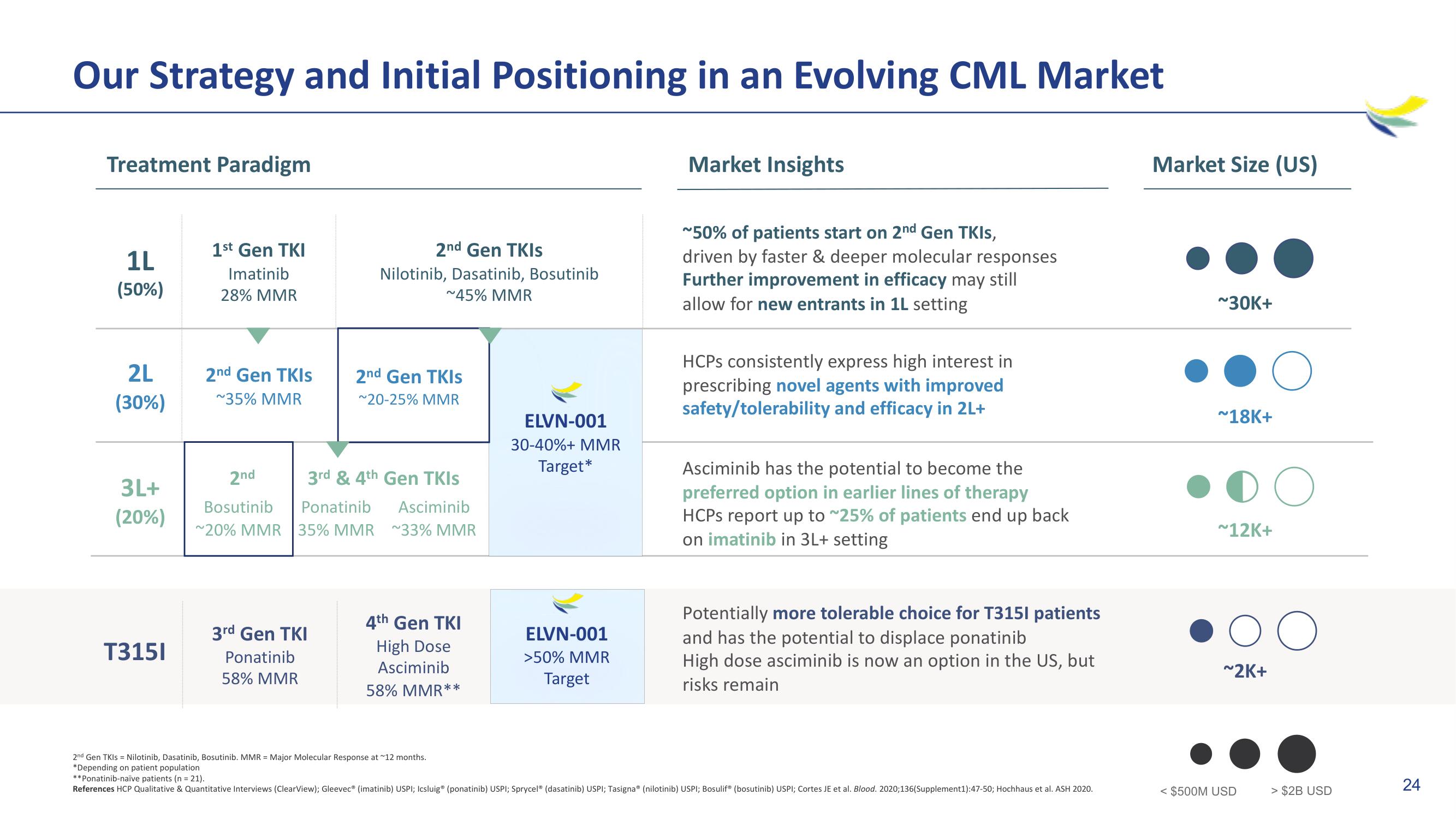
Our Strategy and Initial Positioning in an Evolving CML Market
Treatment Paradigm
1L
(50%)
2L
(30%)
3L+
(20%)
T3151
1st Gen TKI
Imatinib
28% MMR
2nd Gen TKIS
~35% MMR
2nd Gen TKIS
Nilotinib, Dasatinib, Bosutinib
~45% MMR
3rd Gen TKI
Ponatinib
58% MMR
2nd Gen TKIs
~20-25% MMR
2nd
3rd & 4th Gen TKIs
Bosutinib Ponatinib Asciminib
~20% MMR 35% MMR ~33% MMR
4th Gen TKI
High Dose
Asciminib
58% MMR**
2nd Gen TKIs = Nilotinib, Dasatinib, Bosutinib. MMR = Major Molecular Response at ~12 months.
*Depending on patient population
ELVN-001
30-40%+ MMR
Target*
ELVN-001
>50% MMR
Target
Market Insights
~50% of patients start on 2nd Gen TKIS,
driven by faster & deeper molecular responses
Further improvement in efficacy may still
allow for new entrants in 1L setting
HCPs consistently express high interest in
prescribing novel agents with improved
safety/tolerability and efficacy in 2L+
Asciminib has the potential to become the
preferred option in earlier lines of therapy
HCPs report up to ~25% of patients end up back
on imatinib in 3L+ setting
Potentially more tolerable choice for T3151 patients
and has the potential to displace ponatinib
High dose asciminib is now an option in the US, but
risks remain
**Ponatinib-naïve patients (n = 21).
References HCP Qualitative & Quantitative Interviews (ClearView); Gleevec® (imatinib) USPI; Icsluig (ponatinib) USPI; Sprycel® (dasatinib) USPI; Tasigna® (nilotinib) USPI; Bosulif® (bosutinib) USPI; Cortes JE et al. Blood. 2020;136(Supplement1):47-50; Hochhaus et al. ASH 2020.
Market Size (US)
~30K+
O
~18K+
O
~12K+
< $500M USD
оо
~2K+
> $2B USD
24View entire presentation