Neumora Therapeutics IPO Presentation Deck
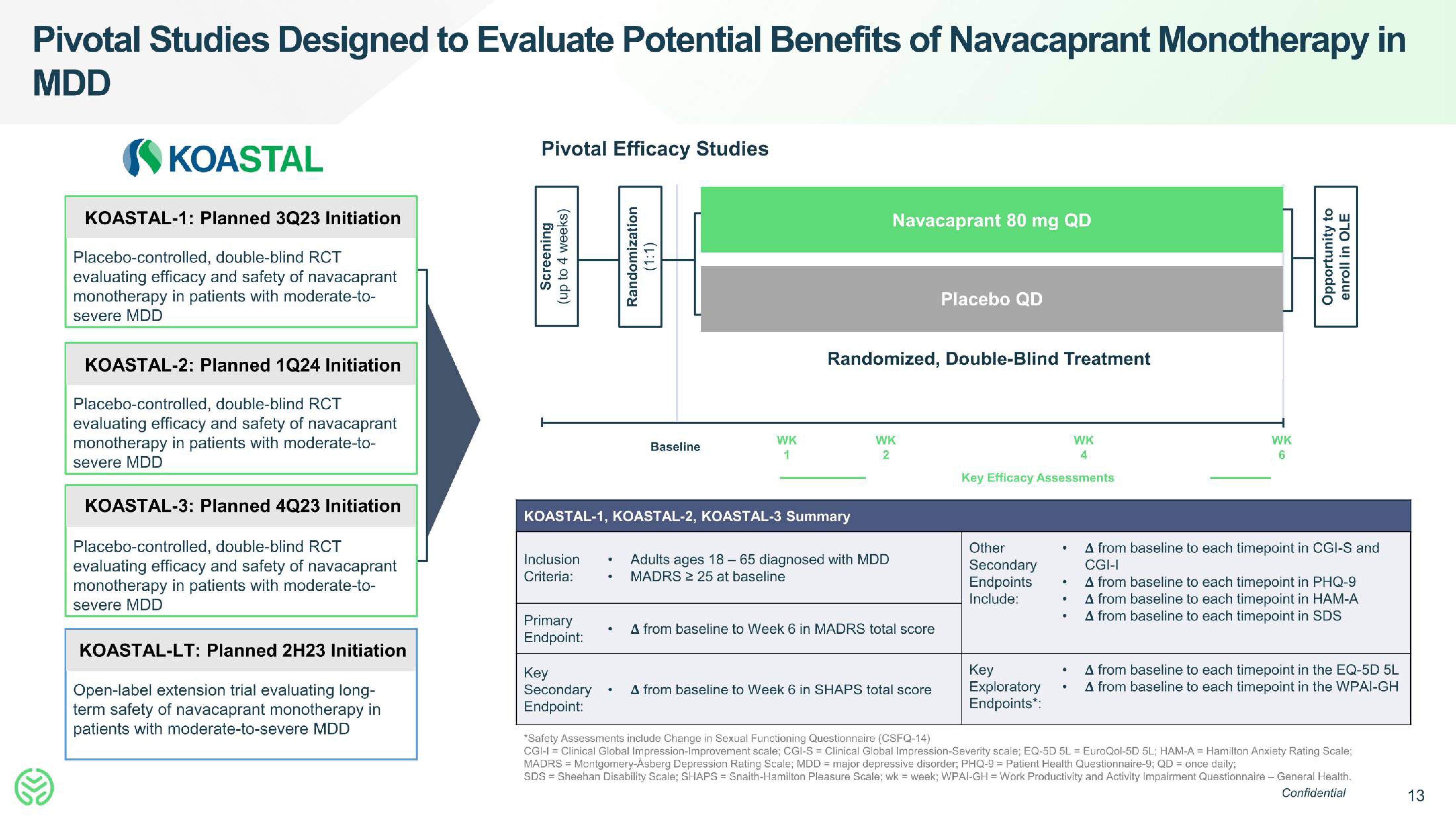
Pivotal Studies Designed to Evaluate Potential Benefits of Navacaprant Monotherapy in
MDD
KOASTAL
KOASTAL-1: Planned 3Q23 Initiation
Placebo-controlled, double-blind RCT
evaluating efficacy and safety of navacaprant
monotherapy in patients with moderate-to-
severe MDD
KOASTAL-2: Planned 1Q24 Initiation
Placebo-controlled, double-blind RCT
evaluating efficacy and safety of navacaprant
monotherapy in patients with moderate-to-
severe MDD
KOASTAL-3: Planned 4Q23 Initiation
Placebo-controlled, double-blind RCT
evaluating efficacy and safety of navacaprant
monotherapy in patients with moderate-to-
severe MDD
KOASTAL-LT: Planned 2H23 Initiation
Open-label extension trial evaluating long-
term safety of navacaprant monotherapy in
patients with moderate-to-severe MDD
Pivotal Efficacy Studies
H
Screening
(up to 4 weeks)
Inclusion
Criteria:
Primary
Endpoint:
Key
Secondary
Endpoint:
Randomization
.
(1:1)
KOASTAL-1, KOASTAL-2, KOASTAL-3 Summary
Baseline
WK
1
Navacaprant 80 mg QD
Randomized, Double-Blind Treatment
WK
2
Adults ages 18-65 diagnosed with MDD
MADRS ≥ 25 at baseline
A from baseline to Week 6 in MADRS total score
Placebo QD
A from baseline to Week 6 in SHAPS total score
WK
4
Key Efficacy Assessments
Other
Secondary
Endpoints
Include:
Key
Exploratory
Endpoints*:
●
●
.
WK
6
Opportunity to
enroll in OLE
A from baseline to each timepoint in CGI-S and
CGI-I
A from baseline to each timepoint in PHQ-9
A from baseline to each timepoint in HAM-A
A from baseline to each timepoint in SDS
A from baseline to each timepoint in the EQ-5D 5L
A from baseline to each timepoint in the WPAI-GH
*Safety Assessments include Change in Sexual Functioning Questionnaire (CSFQ-14)
CGI-I = Clinical Global Impression-Improvement scale; CGI-S = Clinical Global Impression-Severity scale; EQ-5D 5L = EuroQol-5D 5L; HAM-A = Hamilton Anxiety Rating Scale;
MADRS = Montgomery-Åsberg Depression Rating Scale; MDD = major depressive disorder; PHQ-9 = Patient Health Questionnaire-9; QD = once daily;
SDS Sheehan Disability Scale; SHAPS = Snaith-Hamilton Pleasure Scale; wk = week; WPAI-GH = Work Productivity and Activity Impairment Questionnaire - General Health.
Confidential
13View entire presentation