Evotec Results Presentation Deck
evotec
Positive outcome of Phase IIb trial (PAGANINI)
Statistically significant improvement in 24-hour
cough counts per hour over placebo after 12
weeks of treatment²)
• Positive outcome in chronic cough regarding
efficacy, safety and differentiation³)
• Favourable safety and tolerability profile
PAGE 25
Positive Phase IIb headline data supports best-in-class potential
P2X3 antagonist - eliapixant (BAY1817080) Refractory Chronic Cough (RCC)
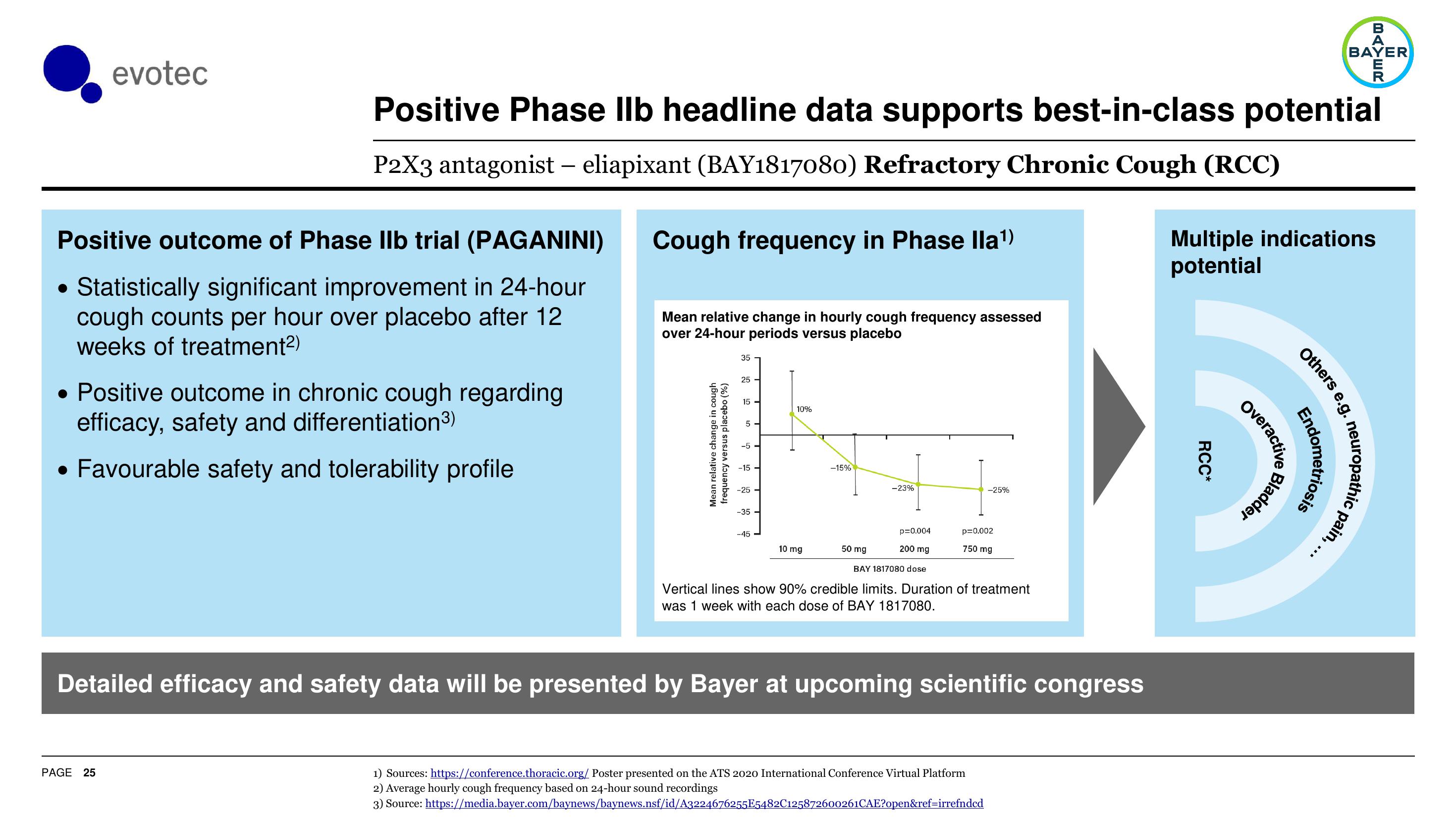
Cough frequency in Phase lla¹)
Mean relative change in hourly cough frequency assessed
over 24-hour periods versus placebo
Mean relative change in cough
frequency versus placebo (%)
35
25
15
5
-5
-15
-25
-35
10%
-45
-15%
10 mg
p=0.004
200 mg
BAY 1817080 dose
Vertical lines show 90% credible limits. Duration of treatment
was 1 week with each dose of BAY 1817080.
-23%
50 mg
-25%
p=0.002
750 mg
Detailed efficacy and safety data will be presented by Bayer at upcoming scientific congress
1) Sources: https://conference.thoracic.org/ Poster presented on the ATS 2020 International Conference Virtual Platform
2) Average hourly cough frequency based on 24-hour sound recordings
3) Source: https://media.bayer.com/baynews/baynews.nsf/id/A3224676255E5482C125872600261CAE?open&ref=irrefndcd
RCC*
Multiple indications
potential
Overactive
Bladder
Others
BAYER
BAYER
Endometriosis
neuropathic
...
pain,View entire presentation