Immix Biopharma Investor Presentation Deck
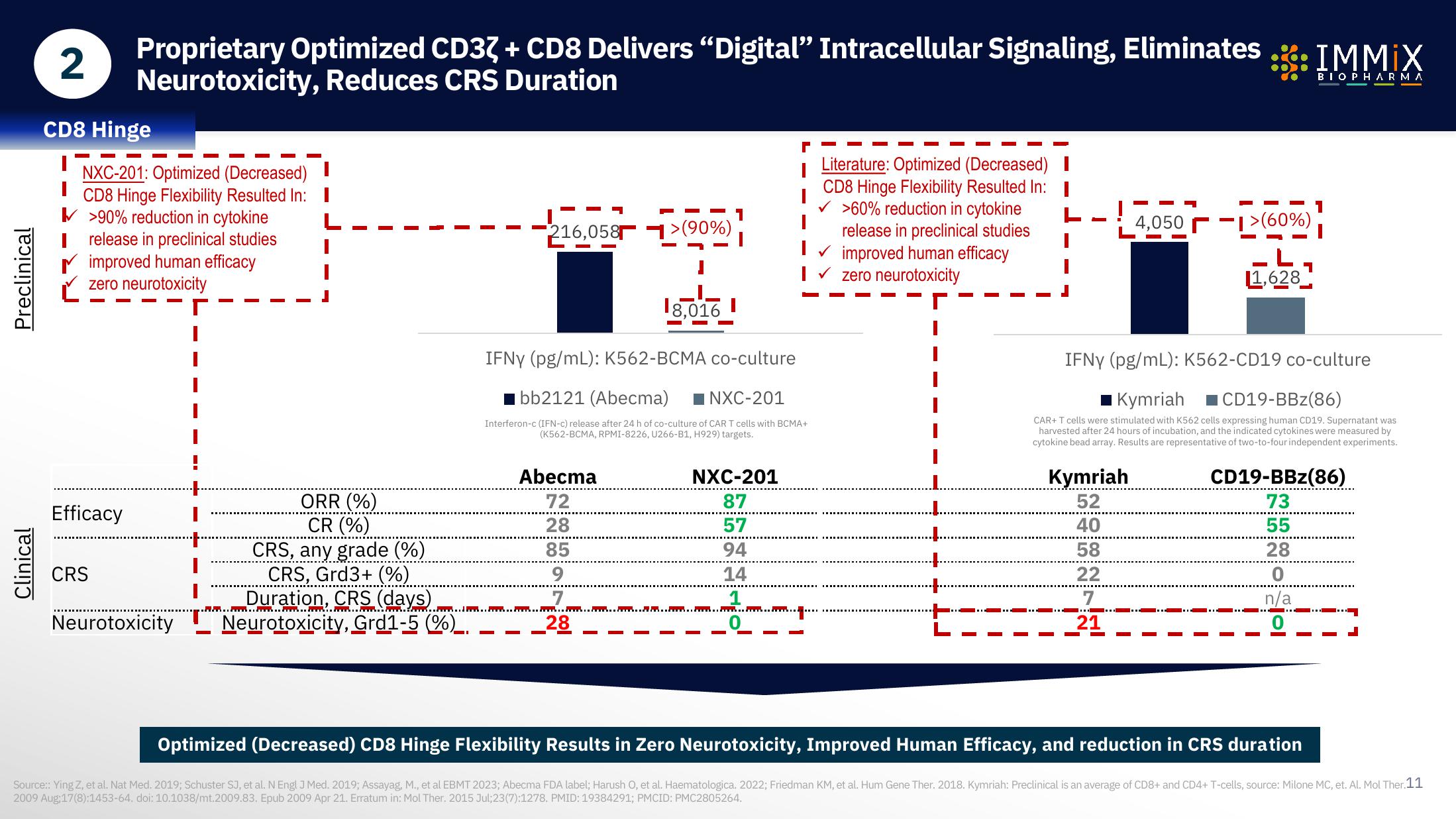
Preclinical
Clinical
2
CD8 Hinge
NXC-201: Optimized (Decreased)
CD8 Hinge Flexibility Resulted In: I
>90% reduction in cytokine
Irelease in preclinical studies
improved human efficacy
zero neurotoxicity
Efficacy
Proprietary Optimized CD33+ CD8 Delivers "Digital” Intracellular Signaling, Eliminates
Neurotoxicity, Reduces CRS Duration
CRS
Neurotoxicity
ORR (%)
CR (%)
CRS, any grade (%)
CRS, Grd3+ (%)
Duration, CRS (days)
216,058>(90%)
Abecma
72
28
85
9
18,016
IFNY (pg/mL): K562-BCMA co-culture
bb2121 (Abecma) NXC-201
Interferon-c (IFN-c) release after 24 h of co-culture of CAR T cells with BCMA+
(K562-BCMA, RPMI-8226, U266-B1, H929) targets.
28
NXC-201
87
57
94
14
1
AM.............….....…..
Neurotoxicity, Grd1-5 (%)__
Literature: Optimized (Decreased) I
CD8 Hinge Flexibility Resulted In: I
✓ >60% reduction in cytokine
release in preclinical studies
I✓ improved human efficacy
I✓ zero neurotoxicity
●●●
S
Kymriah
52
40
58
22
7
21
4,0501>(60%)
->(60%)
L
11,628
IMMIX
BIOPHARMA
IFNY (pg/mL): K562-CD19 co-culture
Kymriah CD19-BBz(86)
CAR+ T cells were stimulated with K562 cells expressing human CD19. Supernatant was
harvested after 24 hours of incubation, and the indicated cytokines were measured by
cytokine bead array. Results are representative of two-to-four independent experiments.
CD19-BBz(86)
73
55
28
0
n/a
Optimized (Decreased) CD8 Hinge Flexibility Results in Zero Neurotoxicity, Improved Human Efficacy, and reduction in CRS duration
Source:: Ying Z, et al. Nat Med. 2019; Schuster SJ, et al. N Engl J Med. 2019; Assayag, M., et al EBMT 2023; Abecma FDA label; Harush O, et al. Haematologica. 2022; Friedman KM, et al. Hum Gene Ther. 2018. Kymriah: Preclinical is an average of CD8+ and CD4+ T-cells, source: Milone MC, et. Al. Mol Ther.11
2009 Aug;17(8):1453-64. doi: 10.1038/mt.2009.83. Epub 2009 Apr 21. Erratum in: Mol Ther. 2015 Jul;23(7):1278, PMID: 19384291; PMCID: PMC2805264.View entire presentation