BioAtla Investor Presentation Deck
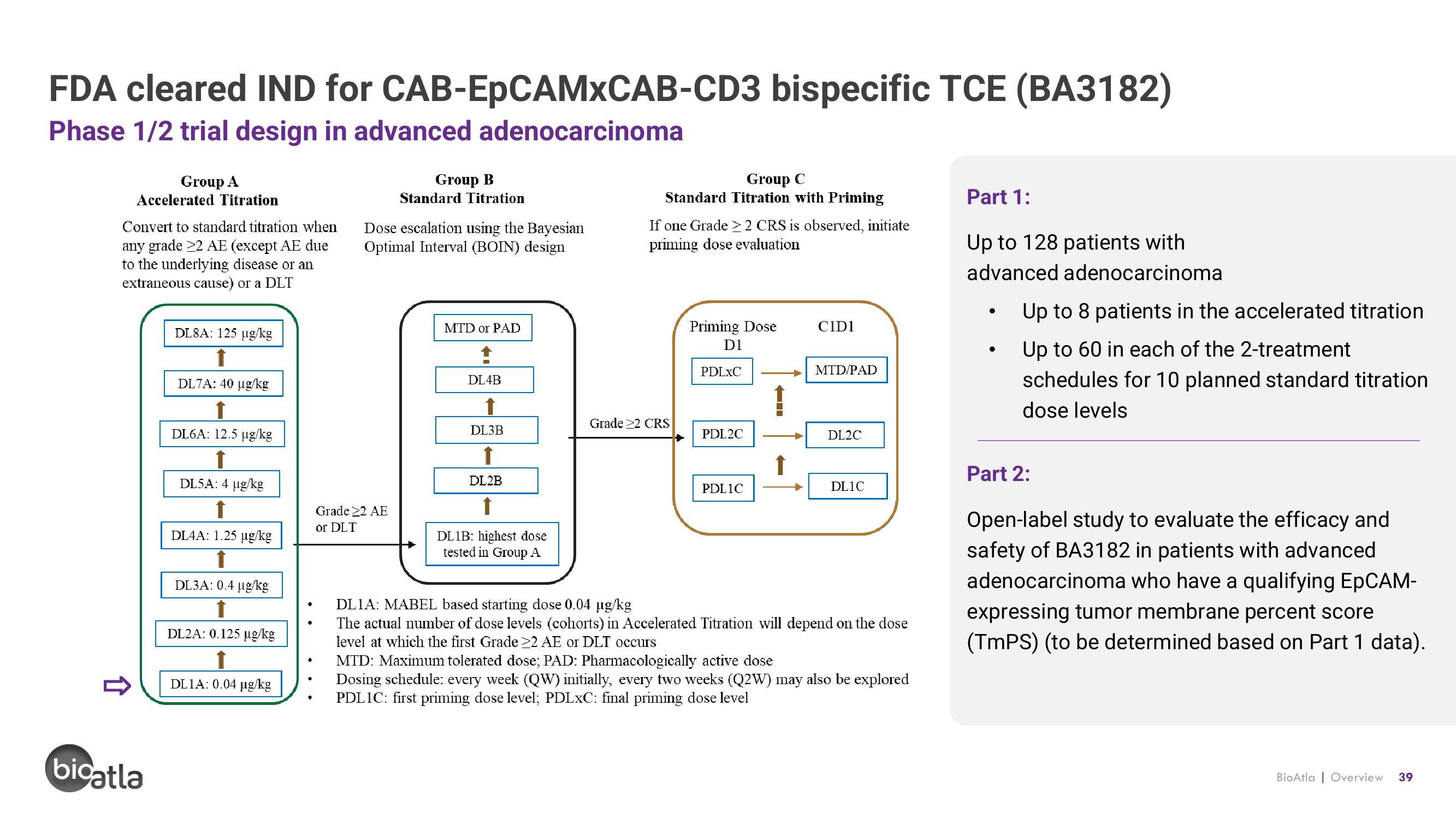
FDA cleared IND for
Phase 1/2 trial design in advanced adenocarcinoma
Group A
Accelerated Titration
Convert to standard titration when
any grade 22 AE (except AE due
to the underlying disease or an
extraneous cause) or a DLT
bicatla
DL8A: 125 µg/kg
DL7A: 40 µg/kg
DL6A: 12.5 µg/kg
↑
DL5A: 4 µg/kg
DL4A: 1.25 µg/kg
DL3A: 0.4 µg/kg
DL2A: 0.125 µg/kg
DL1A: 0.04 µg/kg
CAB-EpCAMXCAB-CD3 bispecific TCE (BA3182)
•
Group B
Standard Titration
Dose escalation using the Bayesian
Optimal Interval (BOIN) design
Grade 22 AE
or DLT
MTD or PAD
DL4B
DL3B
↑
DL2B
↑
DL1B: highest dose
tested in Group A
Group C
Standard Titration with Priming
If one Grade > 2 CRS is observed, initiate
priming dose evaluation
Grade >2 CRS
Priming Dose
D1
PDLXC
PDL2C
PDL1C
1
↑
CIDI
MTD/PAD
DL2C
DL1C
DL1A: MABEL based starting dose 0.04 µg/kg
The actual number of dose levels (cohorts) in Accelerated Titration will depend on the dose
level at which the first Grade 22 AE or DLT occurs
MTD: Maximum tolerated dose; PAD: Pharmacologically active dose
Dosing schedule: every week (QW) initially, every two weeks (Q2W) may also be explored
PDL 1C: first priming dose level; PDLxC: final priming dose level
Part 1:
Up to 128 patients with
advanced adenocarcinoma
●
●
Up to 8 patients in the accelerated titration
Up to 60 in each of the 2-treatment
schedules for 10 planned standard titration
dose levels
Part 2:
Open-label study to evaluate the efficacy and
safety of BA3182 in patients with advanced
adenocarcinoma who have a qualifying EpCAM-
expressing tumor membrane percent score
(TmPS) (to be determined based on Part 1 data).
BioAtla| Overview 39View entire presentation