AstraZeneca Results Presentation Deck
Tagrisso and Imfinzi
Growth improved across the lung cancer franchise
$m
1,400
1,200
1,000
800
600
400
200
0
Q1 2018
Q2 2018
Q3 2018
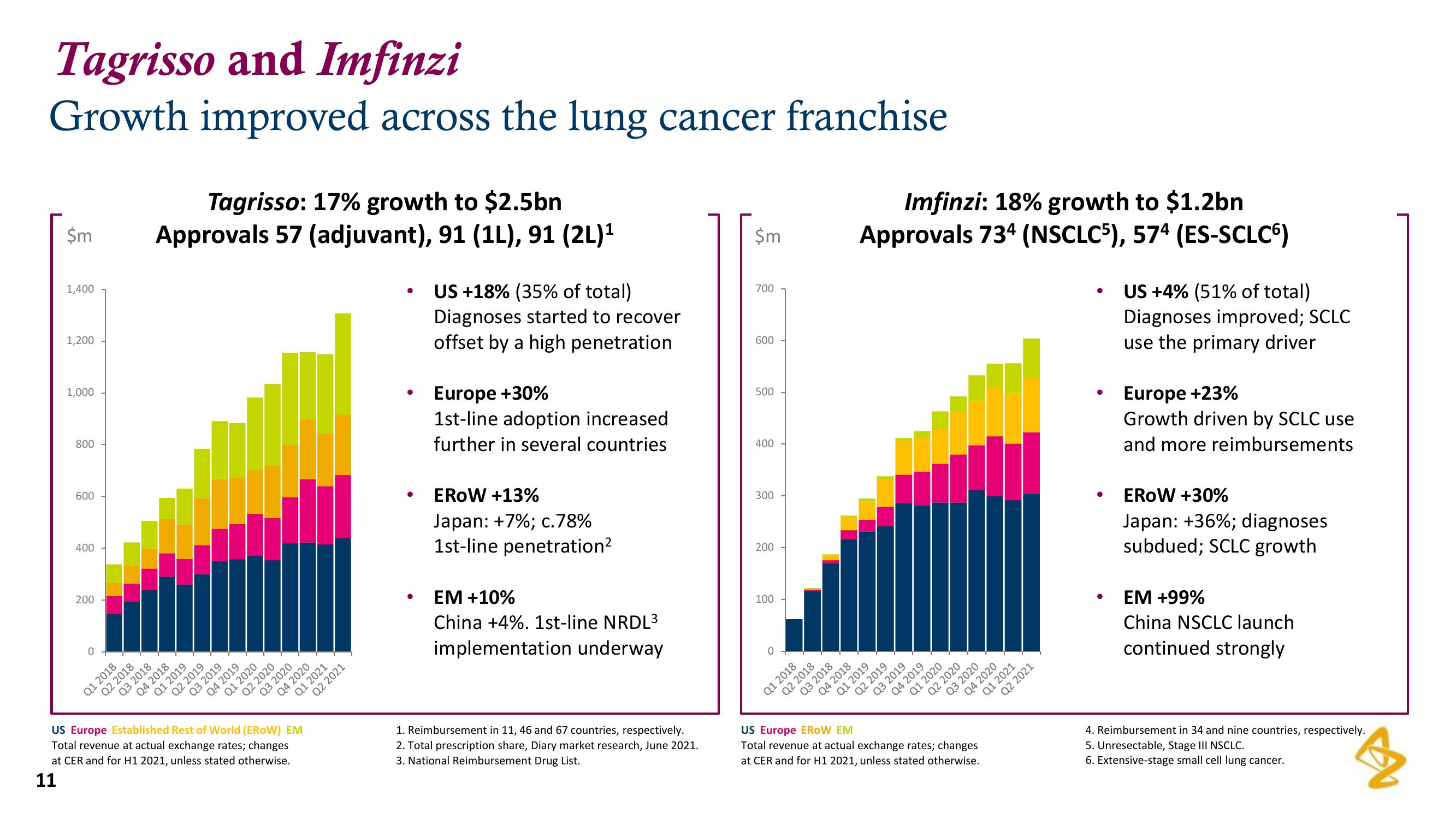
Tagrisso: 17% growth to $2.5bn
Approvals 57 (adjuvant), 91 (1L), 91 (2L)¹
Q4 2018
Q1 2019
Q2 2019
Q3 2019
Q4 2019
Q1 2020
Q2 2020
Q3 2020
US Europe Established Rest of World (EROW) EM
Total revenue at actual exchange rates; changes
at CER and for H1 2021, unless stated otherwise.
11
Q4 2020
Q1 2021
Q2 2021
●
●
●
US +18% (35% of total)
Diagnoses started to recover
offset by a high penetration
Europe +30%
1st-line adoption increased
further in several countries
EROW +13%
Japan: +7%; c.78%
1st-line penetration²
EM +10%
China +4%. 1st-line NRDL³
implementation underway
1. Reimbursement in 11, 46 and 67 countries, respectively.
2. Total prescription share, Diary market research, June 2021.
3. National Reimbursement Drug List.
$m
700
600
500
400
300
200
100
T
T
Q1 2018
Q2 2018
Q3
2018
Q4
Imfinzi: 18% growth to $1.2bn
Approvals 734 (NSCLC5), 574 (ES-SCLC6)
2018
Q1 2019
Q2 2019
Q3
2019
Q4
2019
Q1
2020
Q2 2020
Q3 2020
US Europe
EROW EM
Total revenue at actual
rates; changes
exchange
at CER and for H1 2021, unless stated otherwise.
Q4 2020
Q1 2021
Q2 2021
●
●
●
●
US +4% (51% of total)
Diagnoses improved; SCLC
use the primary driver
Europe +23%
Growth driven by SCLC use
and more reimbursements
EROW +30%
Japan: +36%; diagnoses
subdued; SCLC growth
EM +99%
China NSCLC launch
continued strongly
4. Reimbursement in 34 and nine countries, respectively.
5. Unresectable, Stage III NSCLC.
6. Extensive-stage small cell lung cancer.
gView entire presentation