Genelux Investor Presentation Deck
Validating Industry Collaboration with Newsoara BioPharma Co., Ltd
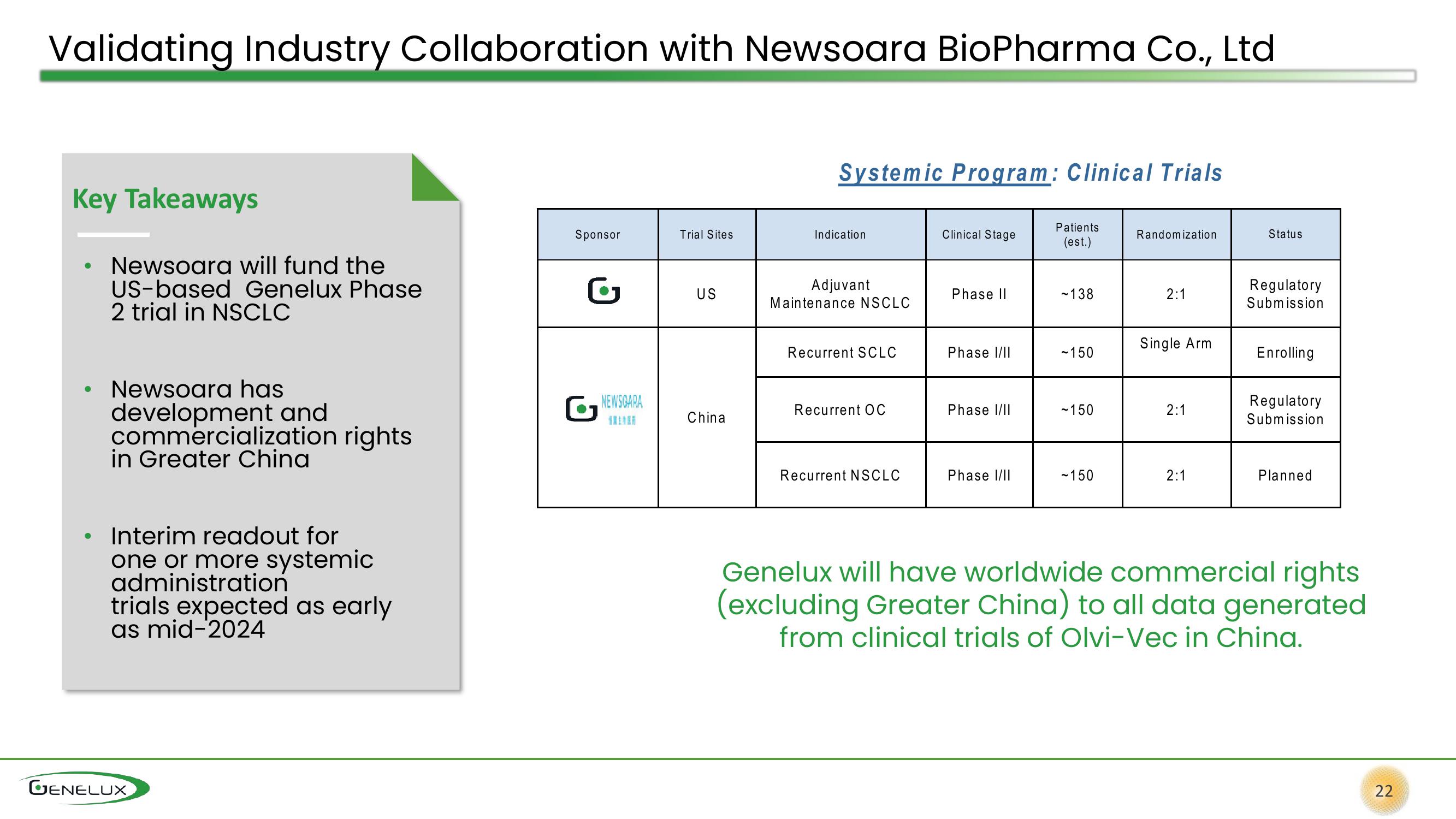
Key Takeaways
• Newsoara will fund the
US-based Genelux Phase
2 trial in NSCLC
• Newsoara has
development and
commercialization rights
in Greater China
• Interim readout for
one or more systemic
administration
trials expected as early
as mid-2024
GENELUX
Sponsor
G
NEWSCARA
Trial Sites
US
China
Systemic Program: Clinical Trials
Indication
Adjuvant
Maintenance NSCLC
Recurrent SCLC
Recurrent OC
Recurrent NSCLC
Clinical Stage
Phase II
Phase I/II
Phase I/II
Phase I/II
Patients
(est.)
~138
-150
~150
~ 150
Randomization
2:1
Single Arm
2:1
2:1
Status
Regulatory
Submission
Enrolling
Regulatory
Submission
Planned
Genelux will have worldwide commercial rights
(excluding Greater China) to all data generated
from clinical trials of Olvi-Vec in China.
22View entire presentation