argenx SE Investor Day Presentation Deck
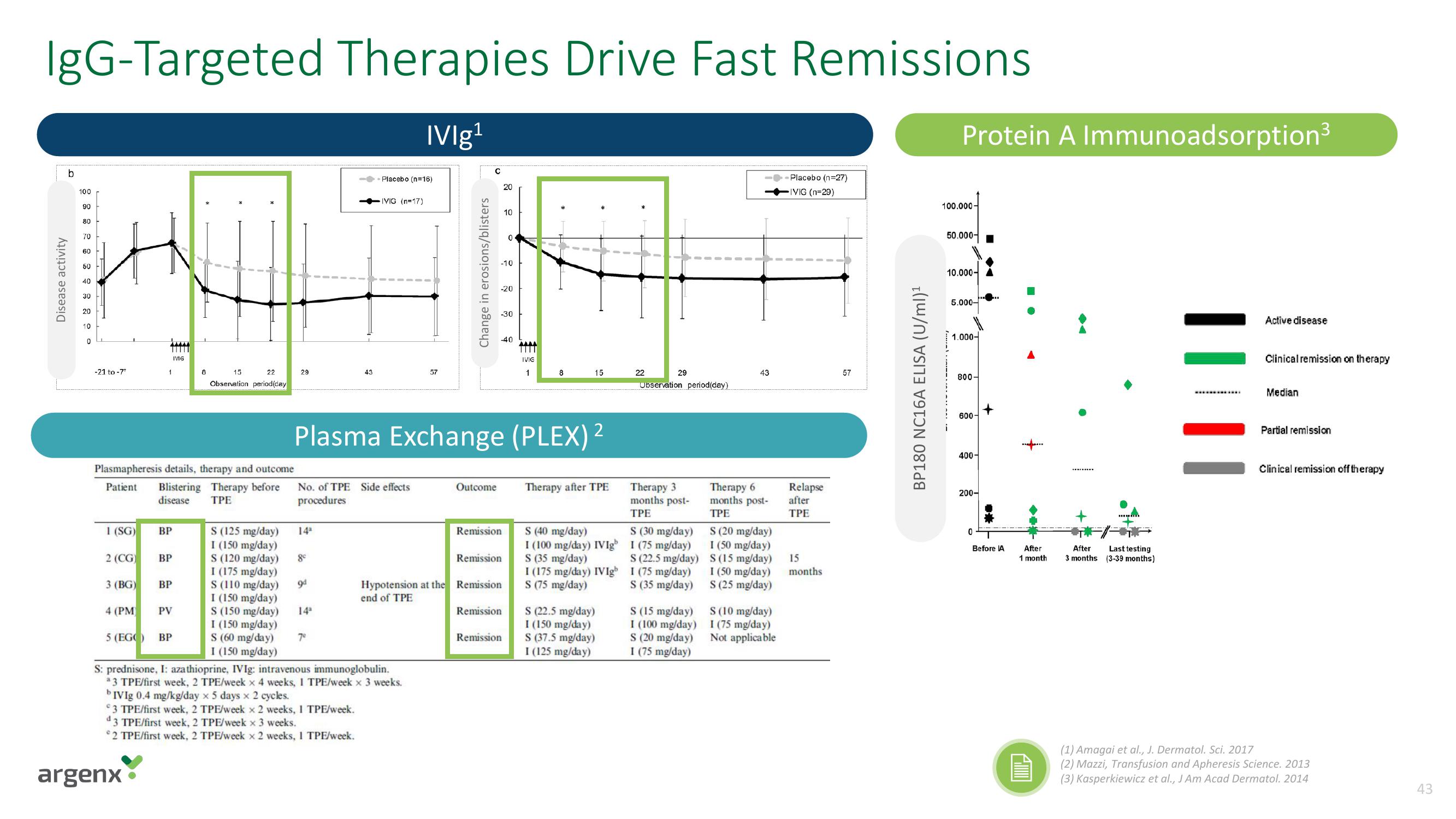
IgG-Targeted Therapies Drive Fast Remissions
IVIg¹
Disease activity
b
100
90
80
70
60
50
40
30
20
10
0
-21 to -7
1 (SG)
2 (CG)
3 (BG)
4 (PM)
Plasmapheresis details, therapy and outcome
Patient
Blistering Therapy before
disease ТРЕ
5 (EG)
*****
IMIG
1
argenx
BP
BP
BP
PV
8
BP
15
22
Observation period (day)
S (125 mg/day)
I (150 mg/day)
S (120 mg/day)
I (175 mg/day)
S (110 mg/day)
I (150 mg/day)
S (150 mg/day)
I (150 mg/day)
S (60 mg/day)
I (150 mg/day)
29
14ª
8º
No. of TPE Side effects
procedures
9d
43
7⁰
Placebo (n=16)
3 TPE/first week, 2 TPE/week x 2 weeks, 1 TPE/week.
3 TPE/first week, 2 TPE/week x 3 weeks.
2 TPE/first week, 2 TPE/week x 2 weeks, 1 TPE/week.
IVIG (n=17)
S: prednisone, I: azathioprine, IVIg: intravenous immunoglobulin.
* 3 TPE/first week, 2 TPE/week x 4 weeks, I TPE/week x 3 weeks.
bIVIg 0.4 mg/kg/day x 5 days x 2 cycles.
57
Change in erosions/blisters
Outcome
Plasma Exchange (PLEX) ²
-10
Remission
20
-20
Remission
10
-30
Hypotension at the Remission
end of TPE
0
-40
Remission
Remission
IVIG
1
8
15
Therapy after TPE
S (40 mg/day)
I (100 mg/day) IVIg
S (35 mg/day)
I (175 mg/day) IVIgb
S (75 mg/day)
S (22.5 mg/day)
I (150 mg/day)
S (37.5 mg/day)
I (125 mg/day)
22
29
Observation period (day)
Therapy 3
months post-
ТРЕ
S (30 mg/day)
I (75 mg/day)
S (22.5 mg/day)
I (75 mg/day)
S (35 mg/day)
S (15 mg/day)
I (100 mg/day)
S (20 mg/day)
1 (75 mg/day)
43
Therapy 6
months post-
TPE
S (20 mg/day)
I (50 mg/day)
S (15 mg/day)
I (50 mg/day)
S (25 mg/day)
S (10 mg/day)
I (75 mg/day)
Not applicable
Placebo (n=27)
-IVIG (n=29)
Relapse
after
TPE
15
months
57
BP180 NC16A ELISA (U/ml)¹
Protein A Immunoadsorption³
100.000-
50.000-
10.000-
5.000-
1.000-
800
600-
400-
200-
0
■
Before IA
After
1 month
G
After
Last testing
3 months (3-39 months)
Active disease
Clinical remission on therapy
Median
Partial remission
Clinical remission off therapy
(1) Amagai et al., J. Dermatol. Sci. 2017
(2) Mazzi, Transfusion and Apheresis Science. 2013
(3) Kasperkiewicz et al., J Am Acad Dermatol. 2014
43View entire presentation