Aravive Investor Presentation Deck
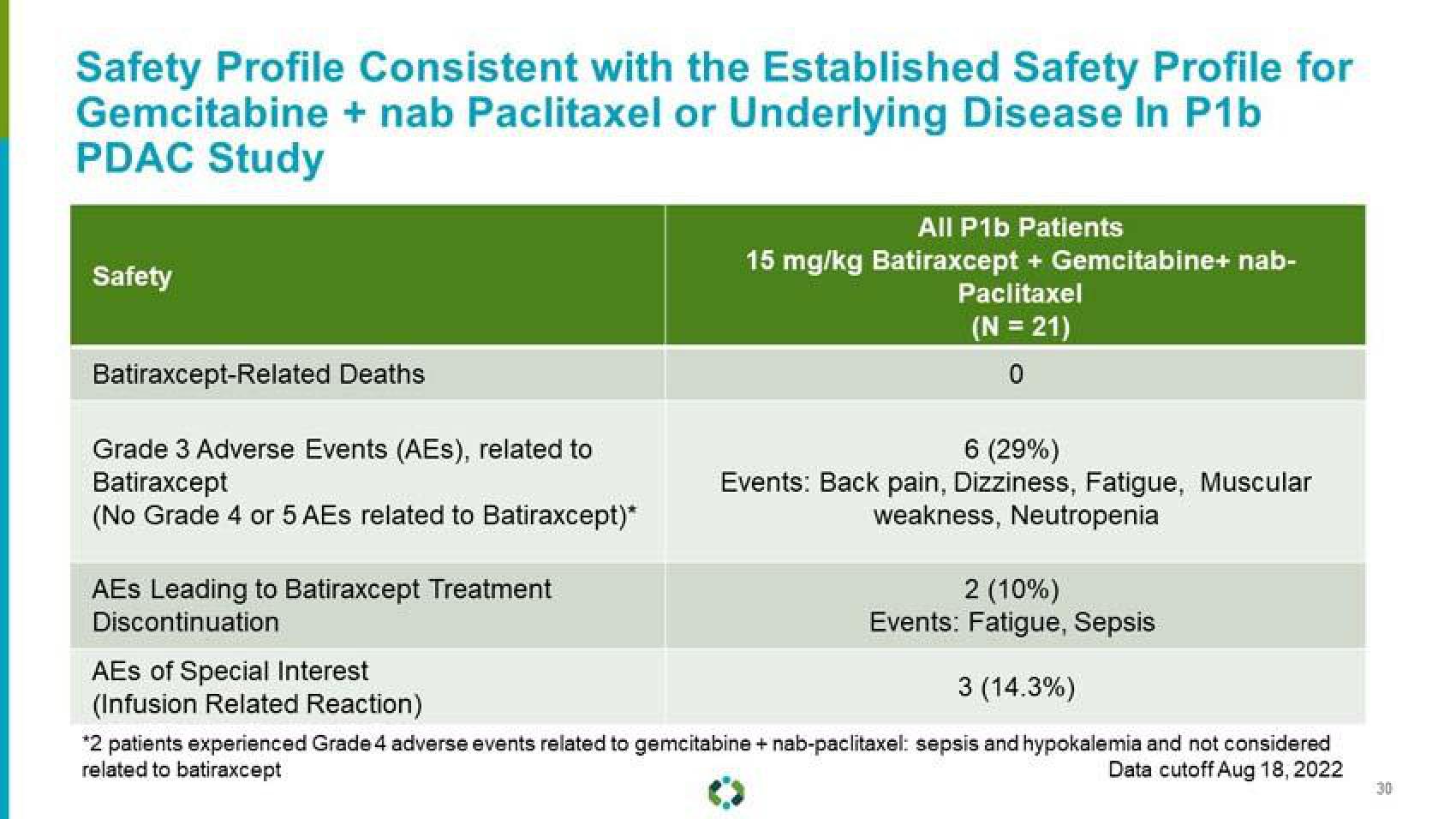
Safety Profile Consistent with the Established Safety Profile for
Gemcitabine + nab Paclitaxel or Underlying Disease In P1b
PDAC Study
Safety
Batiraxcept-Related Deaths
Grade 3 Adverse Events (AEs), related to
Batiraxcept
(No Grade 4 or 5 AEs related to Batiraxcept)*
AEs Leading to Batiraxcept Treatment
Discontinuation
AES of Special Interest
(Infusion Related Reaction)
All P1b Patients
15 mg/kg Batiraxcept + Gemcitabine+ nab-
Paclitaxel
(N = 21)
0
6 (29%)
Events: Back pain, Dizziness, Fatigue, Muscular
weakness, Neutropenia
2 (10%)
Events: Fatigue, Sepsis
3 (14.3%)
*2 patients experienced Grade 4 adverse events related to gemcitabine + nab-paclitaxel: sepsis and hypokalemia and not considered
related to batiraxcept
Data cutoff Aug 18, 2022View entire presentation