Genelux Investor Presentation Deck
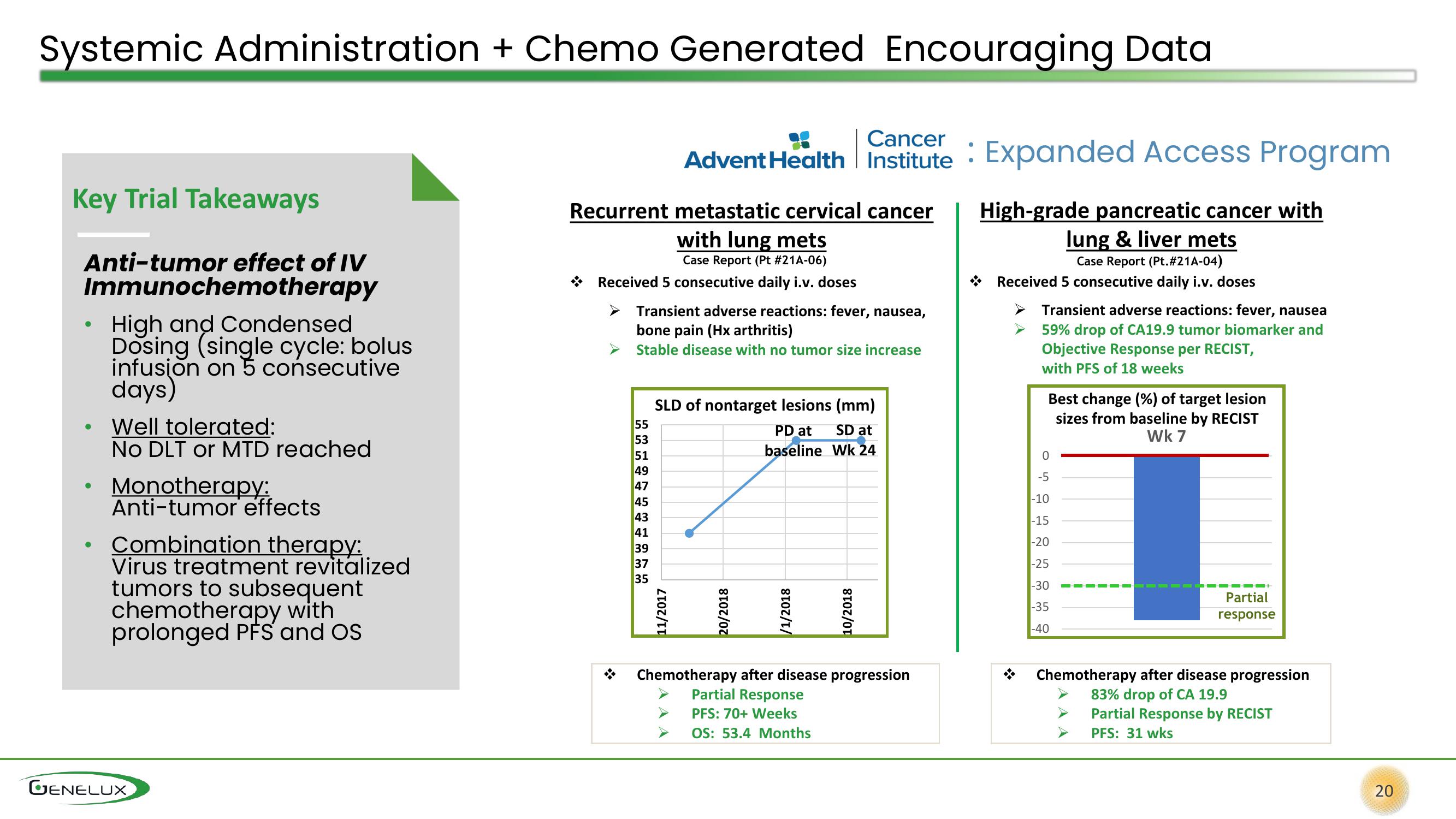
Systemic Administration + Chemo Generated Encouraging Data
Key Trial Takeaways
Anti-tumor effect of IV
Immunochemotherapy
●
●
High and Condensed
Dosing (single cycle: bolus
infusion on 5 consecutive
days)
Well tolerated:
No DLT or MTD reached
Monotherapy:
Anti-tumor effects
Combination therapy:
Virus treatment revitalized
tumors to subsequent
chemotherapy with
prolonged PFS and OS
GENELUX
Recurrent metastatic cervical cancer
with lung mets
Case Report (Pt #21A-06)
Received 5 consecutive daily i.v. doses
Cancer
Advent Health Institute Expanded Access Program
Transient adverse reactions: fever, nausea,
bone pain (Hx arthritis)
Stable disease with no tumor size increase
55
53
51
49
47
45
43
41
39
37
35
SLD of nontarget lesions (mm)
PD at
SD at
baseline Wk 24
11/2017
20/2018
/1/2018
10/2018
Chemotherapy after disease progression
Partial Response
PFS: 70+ Weeks
OS: 53.4 Months
High-grade pancreatic cancer with
lung & liver mets
Case Report (Pt.#21A-04)
Received 5 consecutive daily i.v. doses
Transient adverse reactions: fever, nausea
59% drop of CA19.9 tumor biomarker and
Objective Response per RECIST,
with PFS of 18 weeks
Best change (%) of target lesion
sizes from baseline by RECIST
Wk 7
0
-5
-10
-15
-20
-25
-30
-35
-40
Partial
response
Chemotherapy after disease progression
83% drop of CA 19.9
Partial Response by RECIST
PFS: 31 wks
20View entire presentation