BioNTech Investor Day Presentation Deck
BNT 151, BNT152 + BNT153
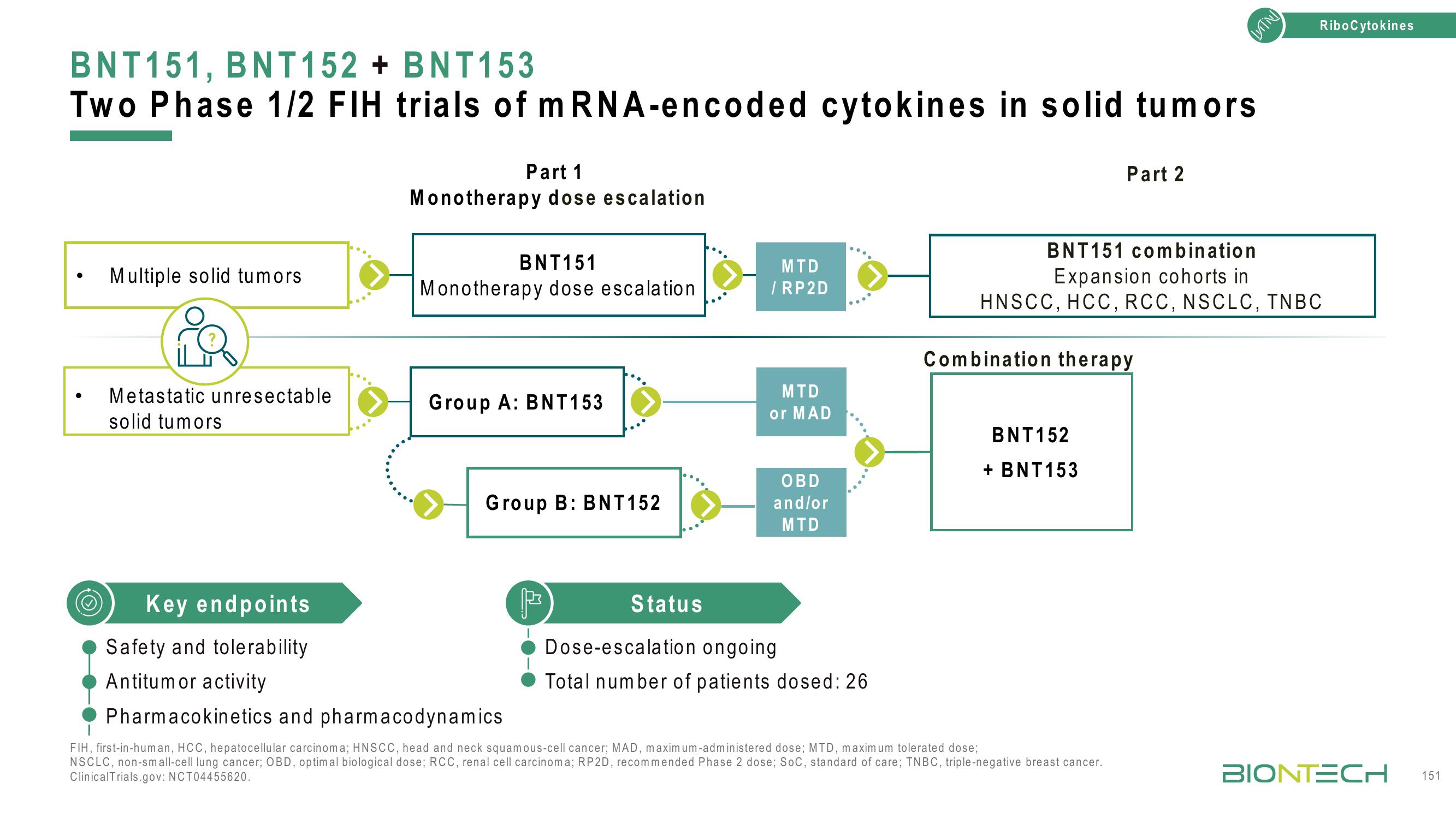
Two Phase 1/2 FIH trials of mRNA-encoded cytokines in solid tumors
●
Ⓒ
Multiple solid tumors
Metastatic unresectable
solid tumors
Key endpoints
Safety and tolerability
Antitumor activity
Part 1
Monotherapy dose escalation
BNT151
Monotherapy dose escalation
Group A: BNT153
Group B: BNT152
3
Status
MTD
/ RP2D
MTD
or MAD
OBD
and/or
MTD
Dose-escalation ongoing
Total number of patients dosed: 26
Part 2
Combination therapy
BNT 152
+ BNT153
BNT151 combination
Expansion cohorts in
HNSCC, HCC, RCC, NSCLC, TNBC
Pharmacokinetics and pharmacodynamics
FIH, first-in-human, HCC, hepatocellular carcinoma; HNSCC, head and neck squamous-cell cancer; MAD, maximum-administered dose; MTD, maximum tolerated dose;
NSCLC, non-small-cell lung cancer; OBD, optimal biological dose; RCC, renal cell carcinoma; RP2D, recommended Phase 2 dose; SoC, standard of care; TNBC, triple-negative breast cancer.
Clinical Trials.gov: NCT04455620.
NUM
RiboCytokines
BIONTECH
151View entire presentation